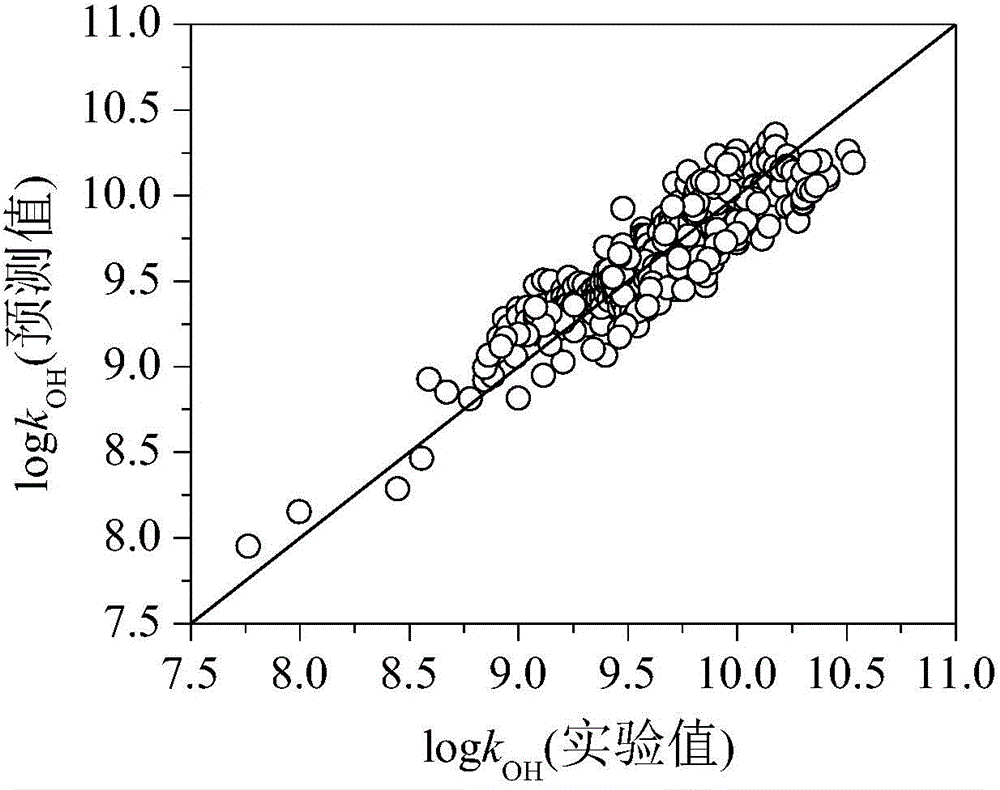
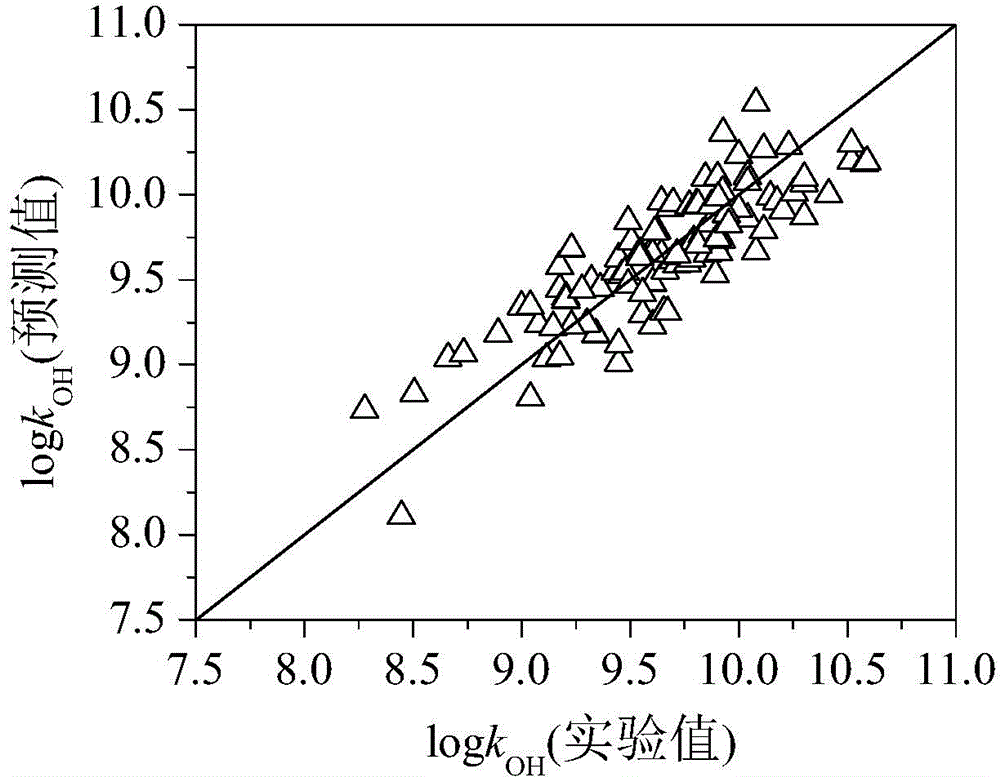
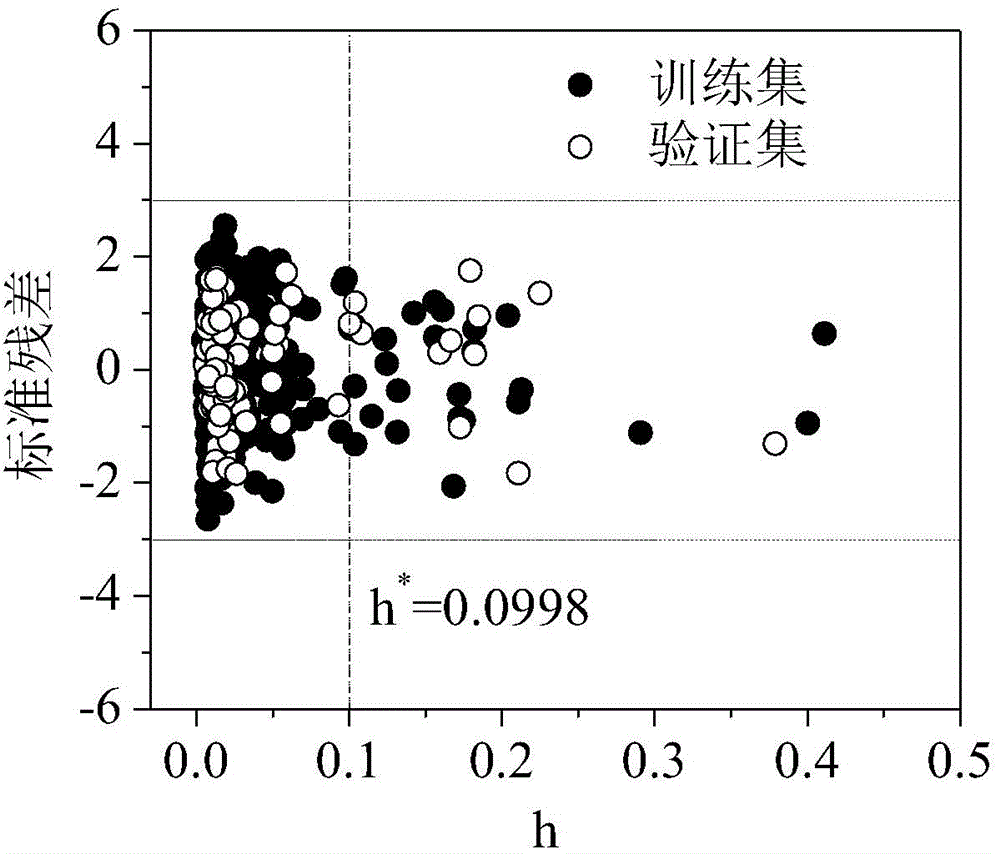
Method for predicting organic compound and hydroxyl radical reaction rate constant in water phase
A technology of reaction rate constants and organic compounds, applied in prediction, data processing applications, instruments, etc., can solve problems such as inconvenient application, insufficient model transparency, difficult model mechanism interpretation, etc., to achieve model algorithm transparency, easy application and promotion, and simple methods. quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] n-heptanol, experimentally determined water phase logk OH with a value of 9.87, the calculated quantum chemistry and Dragon descriptors are: [E HOMO ]=-0.394, [HATS2s]=0.818, [Mor23u]=-1.293, [GATS1e]=1.049, [N-075]=0, [nR=Cp]=0, [nRCONH2]=0, [C-001 ]=1,[MLOGP]=1.940,[nS]=0,[nBR]=0,[q H + ]=0.326, [Eig03_EA(dm)]=0.
[0028] h=0.0171* =0.0998, so the compound is in the application domain, calculated by the model as follows:
[0029] logk OH =6.233[E HOMO [ nS]-0.265[nBR]+0.651[q H + ]+0.119[Eig03_EA(dm)]+11.566
[0030] =6.233×(-0.394)-0.074×0.818-0.183×(-1.293)+0.238×1.049-0.07+0.080×1.940+0.651×0.326+11.566
[0031] =9.83
Embodiment 2
[0033] Benzonitrile, an aromatic nitrogen-containing compound, experimentally determined aqueous phase logk OH With a value of 9.64, the calculated quantum chemistry and Dragon descriptors are: [E HOMO ]=-0.373, [HATS2s]=0.585, [Mor23u]=-0.500, [GATS1e]=0.476, [N-075]=0, [nR=Cp]=0, [nRCONH2]=0, [C-001 ]=0,[MLOGP]=1.769,[nS]=0,[nBR]=0,[q H + ]=0.167, [Eig03_EA(dm)]=0.
[0034] h=0.0171* =0.0998, so the compound is in the application domain, calculated by the model as follows:
[0035] logk OH =6.233[E HOMO [ nS]-0.265[nBR]+0.651[q H + ]+0.119[Eig03_EA(dm)]+11.566
[0036] =6.233×(-0.373)-0.074×0.585-0.183×(-0.500)+0.238×0.476+0.080×1.769+0.651×0.167+11.566
[0037] =9.65
Embodiment 3
[0039] Diisopropyl sulfoxide, S-containing compound, experimentally determined aqueous phase logk OH with a value of 9.83, the calculated quantum chemistry and Dragon descriptors are: [E HOMO ]=-0.314, [HATS2s]=0.864, [Mor23u]=-0.296, [GATS1e]=0.400, [N-075]=0, [nR=Cp]=0, [nRCONH2]=0, [C-001 ]=4,[MLOGP]=1.587,[nS]=1,[nBR]=0,[q H + ]=0.171, [Eig03_EA(dm)]=0.
[0040] h=0.0277* =0.0998, so the compound is in the application domain, calculated by the model as follows:
[0041] logk OH =6.233[E HOMO [ nS]-0.265[nBR]+0.651[q H + ]+0.119[Eig03_EA(dm)]+11.566
[0042] =6.233×(-0.314)-0.074×0.864-0.183×(-0.296)+0.238×0.400+0.070×4+0.080×1.587+0.113+0.651×0.171+11.566
[0043] =9.77
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com