Method for synthesizing phthalate compounds
A technology for ester compounds and phthalic acid, applied in the field of synthesis of phthalic acid ester compounds, can solve the problems of poor stability, difficult to realize industrialization, low catalytic activity, etc., and achieve a simplified separation process and a long time , Improve the conversion rate, the effect of less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
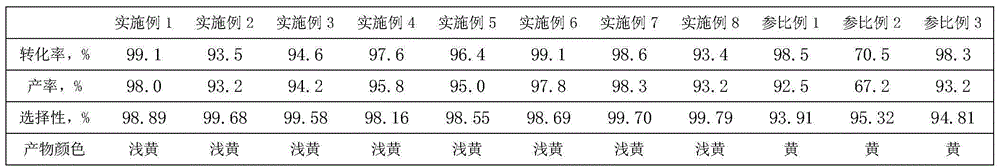
[0024] Add 50 grams of phthalic anhydride in the 250 milliliter three-necked flask with stirring, water separator and reflux condenser, the addition of n-butanol is 2.4 times of the molar number of phthalic anhydride, 0.25 grams of catalyst (by weight In percent, sodium bisulfate: tetrabutylammonium bromide: Polyethylene Glycol=10:1.0:0.1), after heating up to boiling reaction 1h, obtain the catalytic performance of this catalyst with chromatographic analysis: phthalic anhydride conversion ratio The yield of dibutyl phthalate was 99.1%, and the yield of dibutyl phthalate was 98.0%.
Embodiment 2
[0026] Add 50 grams of phthalic anhydride to a 250-milliliter three-neck flask with stirring, water separator and reflux condenser, the addition of n-butanol is 4.0 times the molar number of phthalic anhydride, and 2.0 grams of catalyst (by weight Percentage, potassium hydrogensulfate:polyethylene glycol=10:0.1), after heating up to boiling reaction 2h, obtain the catalytic performance of this catalyst with chromatographic analysis: phthalic anhydride conversion rate is 93.5%, phthalic acid di Butyl ester yield was 93.2%.
Embodiment 3
[0028] Add 50 grams of phthalic anhydride in a 250 ml three-necked flask with stirring, water separator and reflux condenser, the addition of n-butanol is 2.4 times the molar number of phthalic anhydride, 2.5 grams of catalyst (by weight Percentage, sodium bisulfate: tetrabutylammonium bromide=10:0.8), be warming up to boiling reaction after 0.5h, obtain the catalytic performance of this catalyst with chromatographic analysis: phthalic anhydride conversion rate is 94.6%, o-phthalic anhydride The yield of dibutyl dicarboxylate was 94.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com