Preparation method for S-carvedilol
A technology of reaction kettle and ethylamine, applied in the field of preparation of S-carvedilol, can solve the problems of low conversion rate of S-epoxy carbazole, hinder the crystallization and purification of product S-carvedilol, etc., so as to improve the conversion rate. efficiency, continuous production, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The qualitative and quantitative detection method of the reaction substrate and product is: using Kromasil C 18 Column (12.5cm×4.6mm×5μm), mobile phase: acetonitrile: phosphate buffer (pH 2) (35:65); UV detection wavelength 240nm; flow rate: 1.0mL / min; column temperature 55°C.
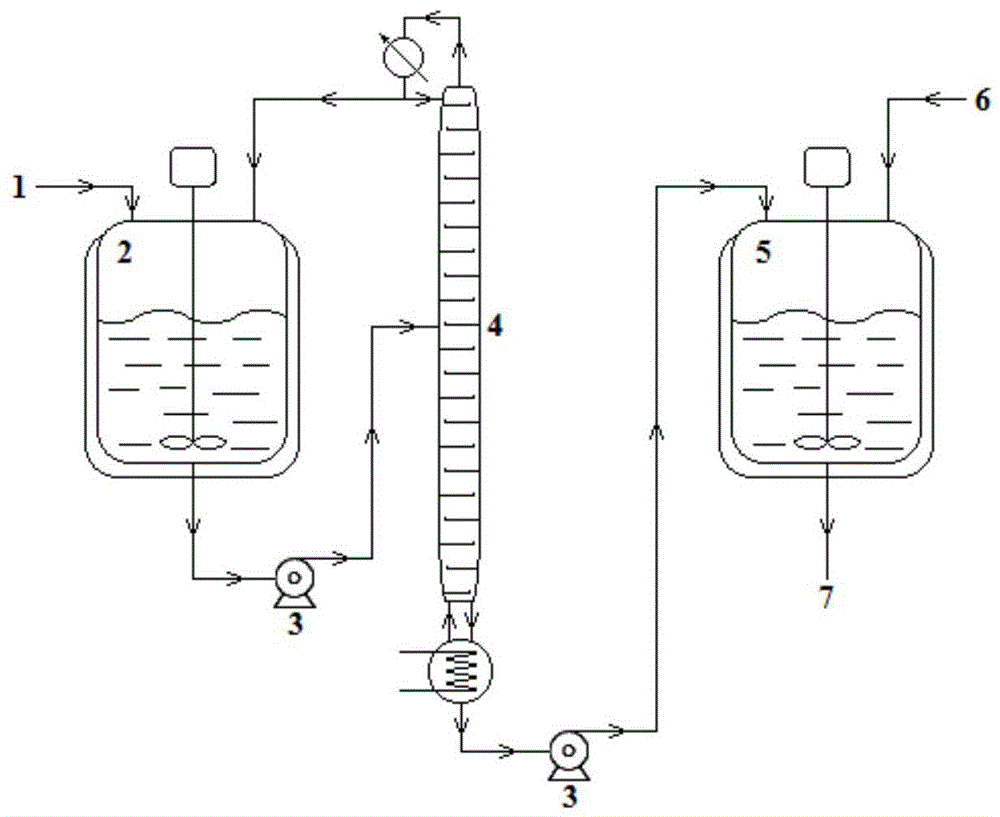
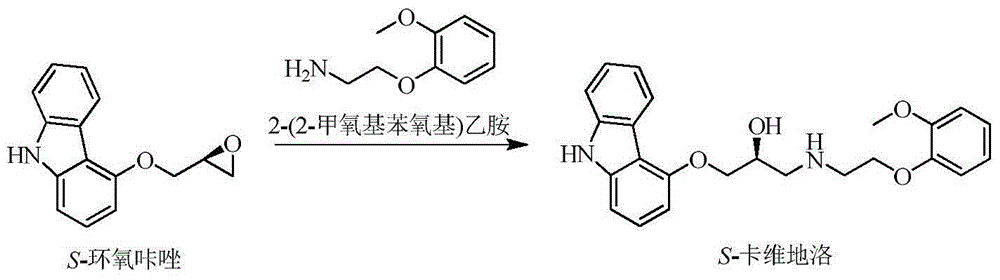
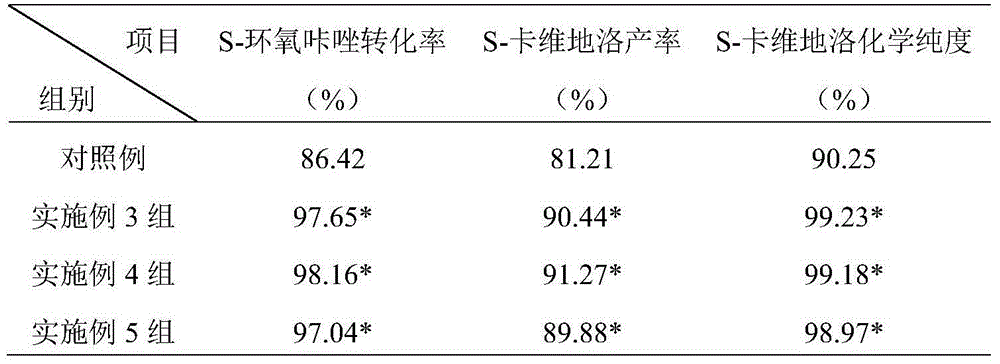
[0024] S-epoxycarbazole, 2-(2-methoxyphenoxy)ethylamine (molar ratio 1:1) and solvent ethanol were pumped into the reaction kettle, heated to a reaction temperature of 80°C, and stirred vigorously for 1 hour. Pump the reaction solution from the bottom of the reaction tank into the middle of the rectification tower, heat it to 90°C for rectification, and separate the product, light ethanol and unreacted 2-(2-methoxyphenoxy)ethylamine at the top of the tower Condensed and refluxed into the reaction kettle, the crude heavy component flowed out from the bottom of the tower into the crystallization kettle, added ethyl acetate and recrystallized at 0°C for 30h to obtain the product. See Table 1 for t...
Embodiment 2
[0026] Reaction substrate and product qualitative and quantitative detection method and operation are all the same as in Example 1, and the implementation steps of changing the reactant molar ratio and each operating parameter are as follows:
[0027] S-epoxycarbazole, 2-(2-methoxyphenoxy)ethylamine (molar ratio 1:12) and solvent ethanol were pumped into the reaction kettle, heated to a reaction temperature of 30°C, and stirred vigorously for 1 hour. Pump the reaction solution from the bottom of the reaction tank into the middle of the rectification tower, heat it to 150°C for rectification, and separate the product, light ethanol and unreacted 2-(2-methoxyphenoxy)ethylamine at the top of the tower Condensed and refluxed into the reaction kettle, the heavy component crude product flowed out from the bottom of the tower into the crystallization kettle, added ethyl acetate and recrystallized at 40°C for 5h to obtain the product. See Table 1 for the results of the conversion rate...
Embodiment 3
[0029] Reaction substrate and product qualitative and quantitative detection method and operation are all the same as in Example 1, and the implementation steps of changing the reactant molar ratio and each operating parameter are as follows:
[0030] Pump S-epoxycarbazole, 2-(2-methoxyphenoxy)ethylamine (molar ratio 1:6.5) and solvent ethanol into the reaction kettle, heat to the reaction temperature of 55°C, and vigorously stir the reaction for 5.5h , the reaction solution is pumped from the bottom of the reaction tank into the middle of the rectification tower, heated to 120°C for rectification, and the product is separated, light ethanol and unreacted 2-(2-methoxyphenoxy)ethylamine The top is condensed, and refluxed into the reaction kettle, the crude heavy component flows out from the bottom of the tower into the crystallization kettle, and ethyl acetate is added to carry out recrystallization at 20°C for 18h to obtain the product. See Table 1 for the results of the conve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com