Outer membrane vesicles
An outer membrane vesicle, heterologous protein technology, applied in allergic diseases, antibody medical ingredients, antibacterial drugs, etc., can solve the problems of no excess, no function, difficult periplasmic protein targeting to OMV, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0229] Example 1-heterologous protein expression into Escherichia coli OMV
[0230] Production of ΔtolR and ΔompA ko Mutants in Escherichia coli BL21(DE3)
[0231] Recombination-prone BL21(DE3) cells were generated by using a highly proficient homologous recombination system (red operon) [122]. Briefly, electrocompetent bacterial cells were transformed by electroporation (5.9 ms at 2.5 kV) with 5 μg of pAJD434 plasmid. Bacteria were then grown in 1 ml of SOC broth for 1 hour at 37°C and then inoculated on LB medium containing trimethoprim (100 μg / ml). Expression of the red gene carried by pAJD434 was induced by adding 0.2% L-arabinose to the medium.
[0232] [Delta]tolR and [Delta]ompA E. coli BL21 mutants known to spontaneously produce large numbers of OMVs were generated by replacing the ompA and tolR coding sequences with kanamycin (kmr) and chloramphenicol (cmr) resistance cassettes, respectively. A three-step PCR protocol was used to fuse the upstream and downstream re...
Embodiment 2
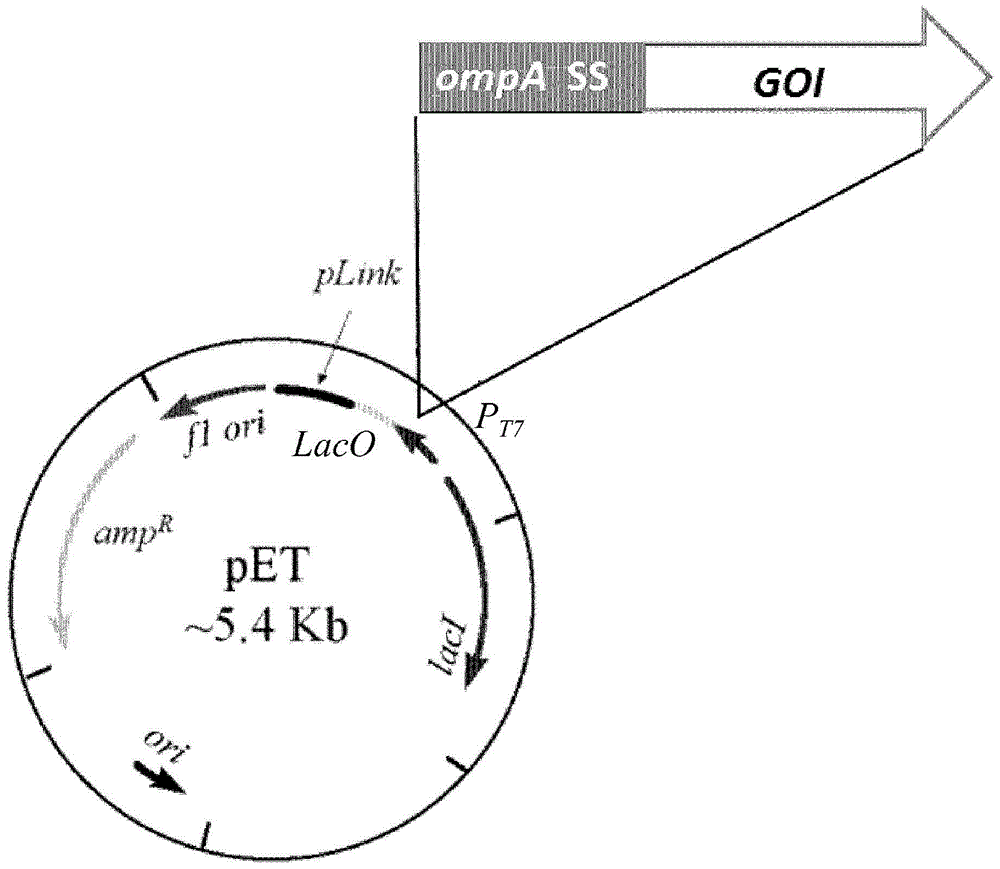
[0238] Example 2 - Plasmid construction
[0239] Five heterologous proteins from different Gram-positive and Gram-negative bacterial species and belonging to different cellular compartments were selected as model proteins to determine whether the heterologous proteins could enter E. coli OMV in their native configuration. These proteins include: (1) periplasmic TEM1 β-lactamase (Bla) from Escherichia coli, (2) factor H-binding protein (fHbp) lipoprotein from Neisseria meningitidis, (3) pyogenic chain Extracellular cholesterol-dependent streptolysin O (Slo, also known as GAS25) from S. pyogenes, (4) the cell envelope serine protease SpyCep from S. pyogenes (also known as GAS57), and (5) S. pyogenes from The putative surface repelling protein Spy0269 (also known as GAS40). The nucleic acid coding sequences for each of these five proteins were cloned into the pET-OmpA plasmid using the polymerase incomplete primer extension (PIPE) cloning method [123]. The pET-OmpA plasmid (als...
Embodiment 3
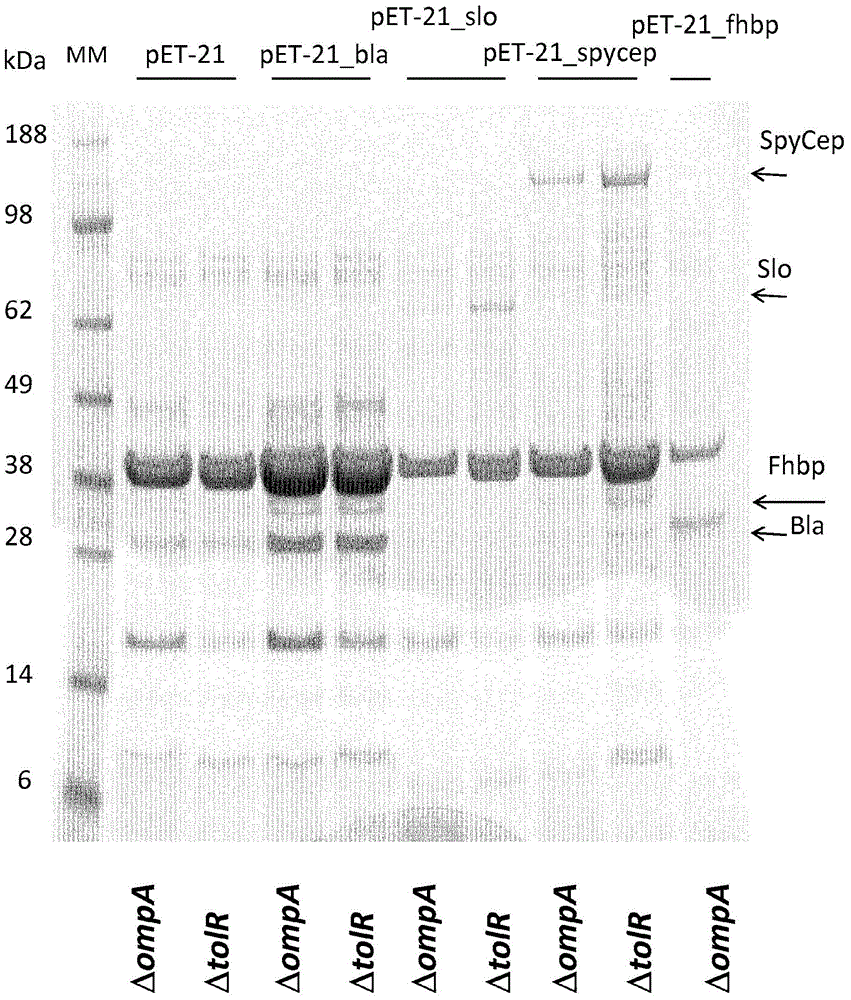
[0241] Example 3 - Expression of heterologous proteins into ΔtolR and ΔompA mutants, OMVs and total lysate preparations
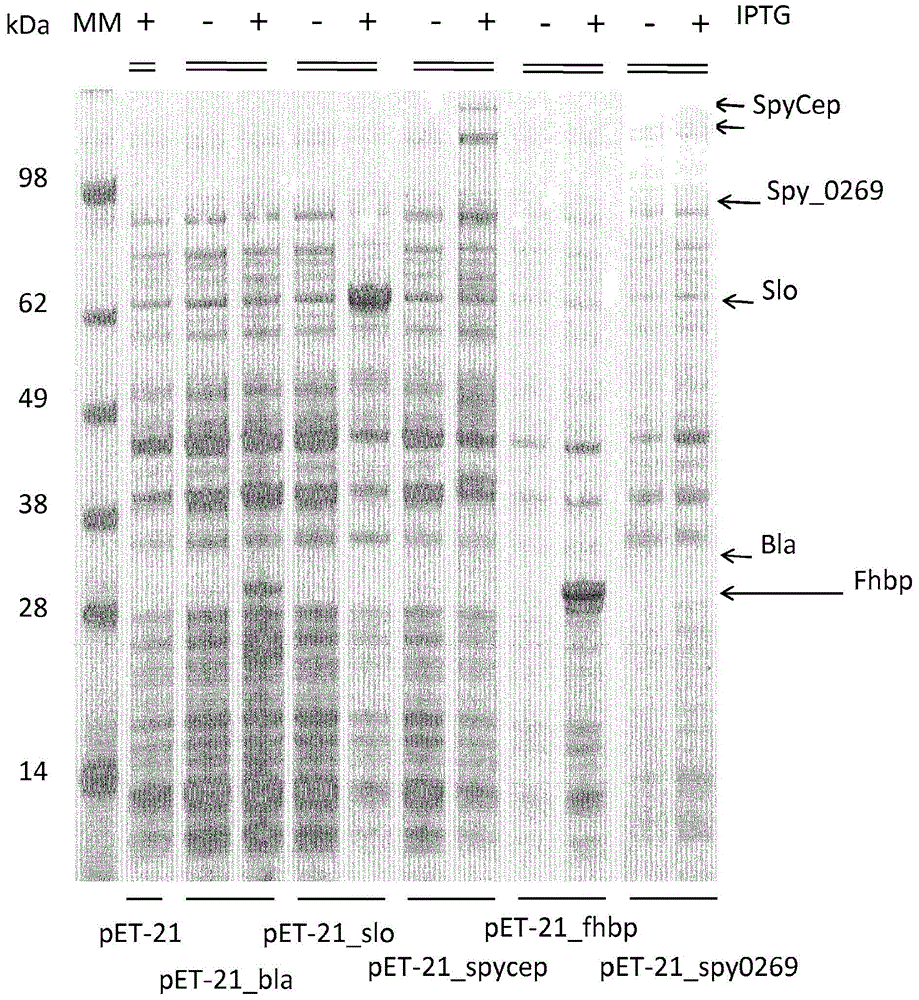
[0242] To investigate whether Bla, slo, SpyCEP, fHbp, and Spy0269 proteins were packaged into OMVs, ΔtolR and ΔompA E. coli BL21 strains were transformed with pET-21_Bla, pET-21_slo, pET-21_SpyCEP, pET-21_fHbp plasmids, and pET21_spy0269. As a negative control, pET-OmpA empty vector was used to transform ΔtolR and ΔompA E. coli BL21 strains.
[0243] All strains were grown to logarithmic phase in liquid medium and induced expression of genes was performed by adding 1 mM IPTG (isopropyl-β-D-thiogalactopyranoside). figure 2 SDS-polyacrylamide gel electrophoresis (SDS-PAGE) of total lysates of these media before and after induction with 1 mM IPTG is shown. Bands corresponding to Bla, slo, SpyCEP, fHbp and Spy0269 proteins were present in all induced samples and indicated by arrows. Thus, all 5 tested heterologous proteins were successfully induced in E. col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com