CHO (Chinese hamster ovary) cell strain for stably expressing human clotting factor VIII and application thereof
A stable expression, human blood coagulation technology, applied in coagulation/fibrinolytic factors, artificial cell constructs, animal cells, etc., can solve problems such as cross-infection and limited plasma sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Use the CHO cell line H9C2 to prepare the eight coagulation factors:
[0030] 1 Experimental method
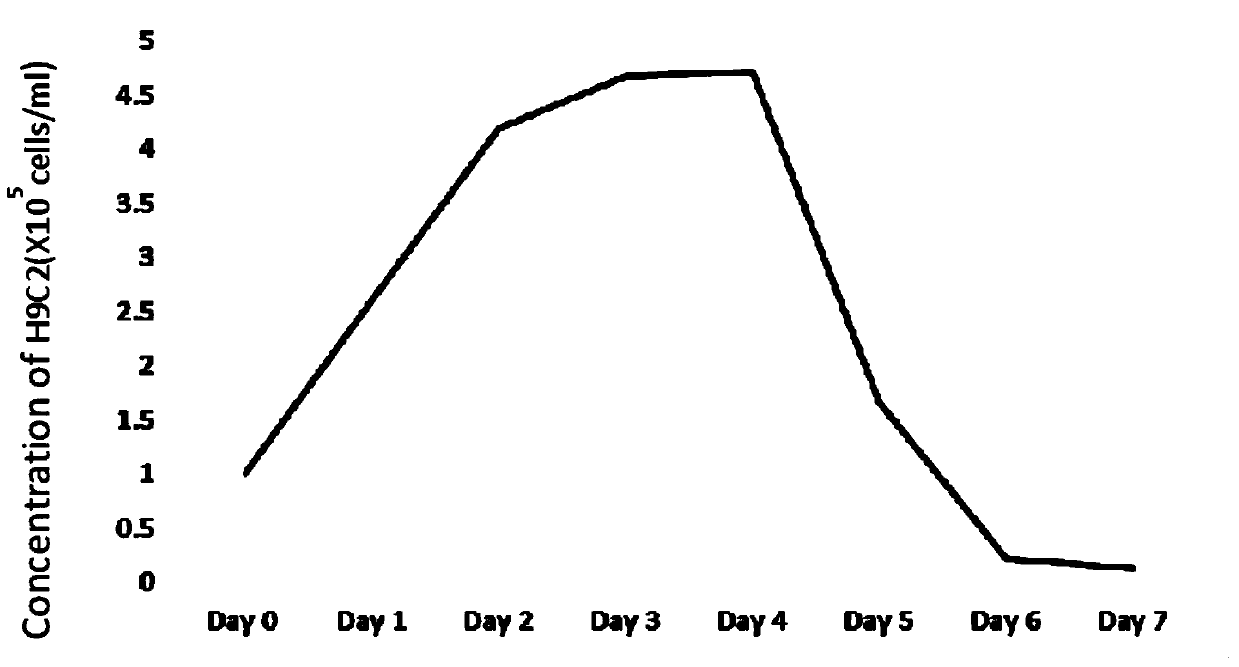
[0031] 7-day continuous culture of CHO cell line H9C2
[0032] The CHO cell line H9C2 with the preservation number of CCTCC No: C2014191 was divided into 1×10 5 Individuals / ml were inoculated into a 2ml system, which was in a 6-well cell culture plate, and the culture medium was CD CHO Serum-Free Medium for CHO Cells, add glutamine at a final concentration of 8mM, and add 20μl Anti-clumping Agent. Since this cell line contains the G418 resistance tag, G-418 Disulfate with a final concentration of 800μg / ml should be added. Count immediately after inoculation to determine the actual inoculum density. The six-well plate was cultured on a shaker in a cell incubator with 37° C., relative humidity of 70-80%, and 5% carbon dioxide at a rotation speed of 125±5 rpm.
[0033]After 2 days of culture (recorded as the first day at the time of inoculation, here is the second da...
Embodiment 2
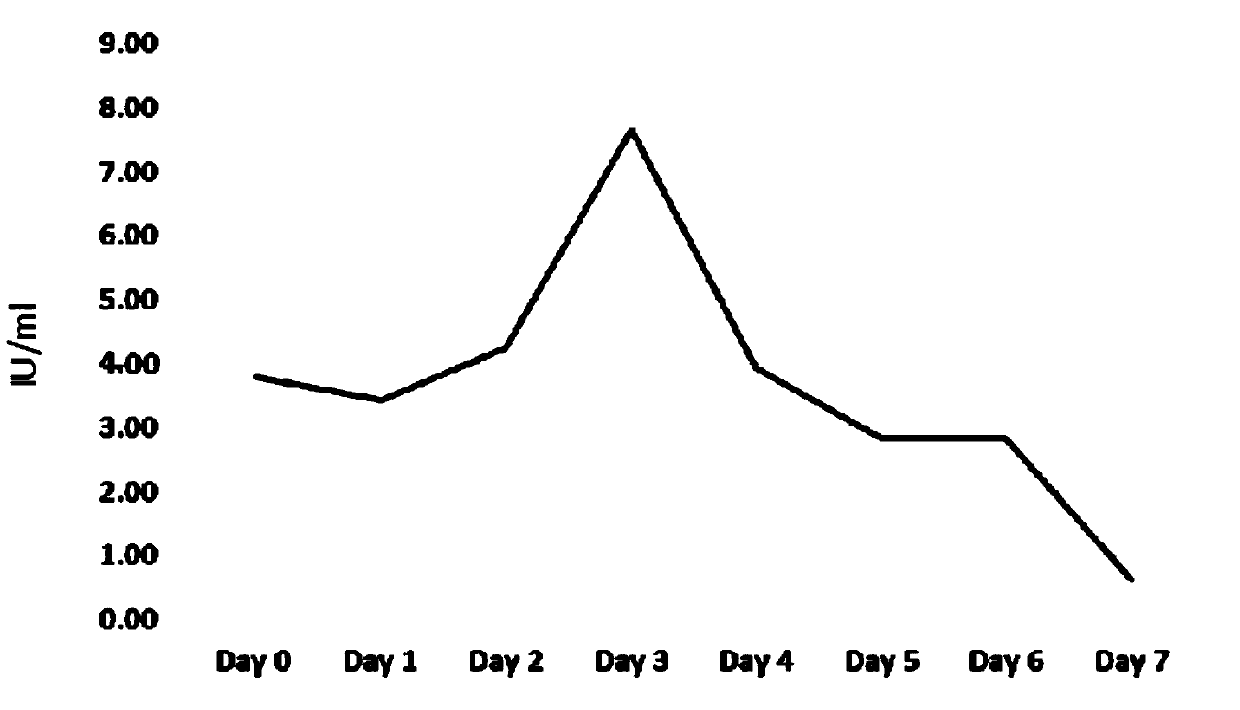
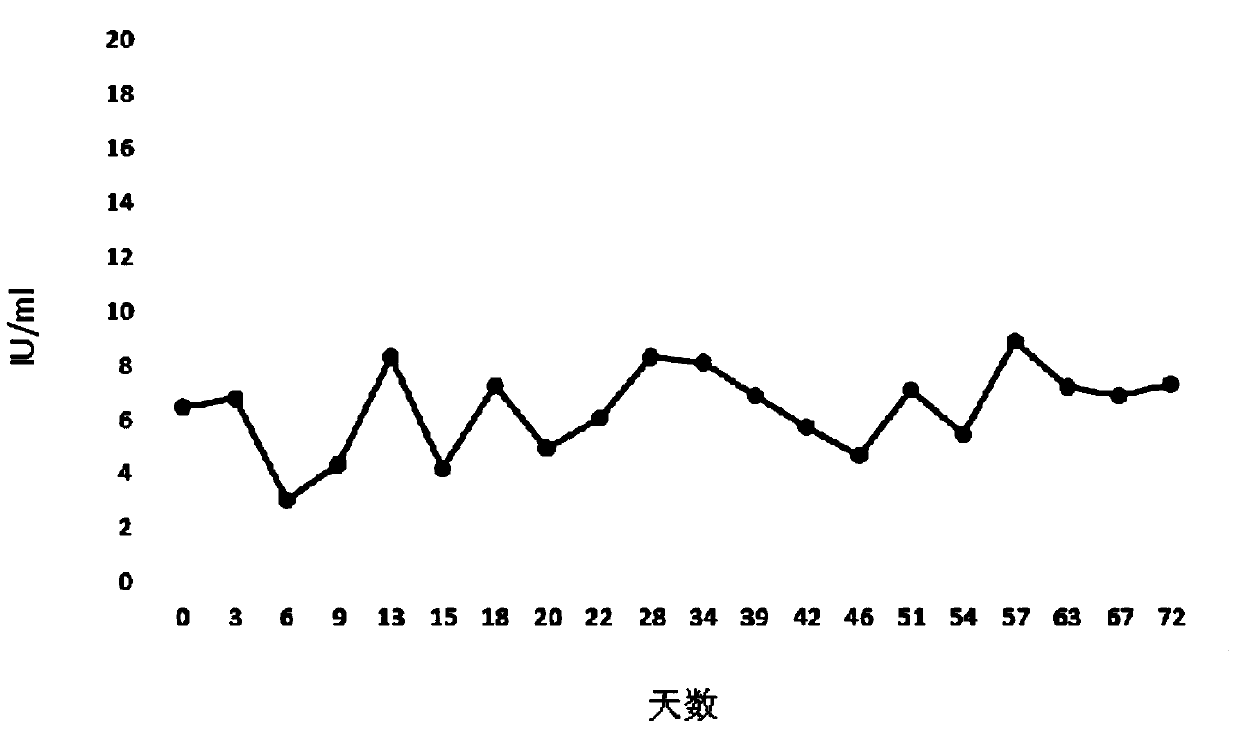
[0039] Stability detection of human coagulation factor 8 expressed in CHO cell line H9C2:
[0040] 1 Experimental method
[0041] (1) Continuous passage of cell lines
[0042] The CHO cell line H9C2 with the preservation number of CCTCC No: C2014191 was divided into 1×10 5 Individuals / ml were inoculated into a 2ml system, which was in a 6-well cell culture plate, and the culture medium was CD CHO Serum-Free Medium for CHO Cells, add glutamine at a final concentration of 8mM, and add 20μl Anti-clumping Agent. Since this cell line contains the G418 resistance tag, G-418Disulfate with a final concentration of 800μg / ml should be added. Placed at 37° C., relative humidity 70-80%, and 5% carbon dioxide cell incubator on a shaker for cultivation, with a rotation speed of 125±5 rpm.
[0043] After 2 days of culture, the cell growth was observed and counted. When counting, first take 50 μL of culture solution (that is, the liquid in which the cells are uniformly dispersed by pipe...
Embodiment 3
[0056] Detection of green fluorescent label in CHO cell line H9C2
[0057] 1 Experimental method
[0058] (1) Fluorescence microscope observation
[0059] In the logarithmic growth phase of the CHO cell line H9C2 (generally 2-3 days after inoculation), the cell suspension was taken out and added to a 10mm cell culture dish (Shanghai Wohong Biotechnology Co., Ltd.), and analyzed with an inverted fluorescence microscope (Olin) Bath, Japan) to observe the green fluorescence. The green fluorescence can be observed at 40 times magnification, and the green fluorescence photo is taken at 100 times magnification, and the white light photo of the same position is taken as a control at the same time.
[0060] Such as Figure 4 Shown is the fluorescent photo of the CHO cell line H9C2 observed under a fluorescent microscope at a magnification of 100 times, and there is a white light photo of the same position as a control. Such as Figure 4 It shows that the cell line is in the logar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com