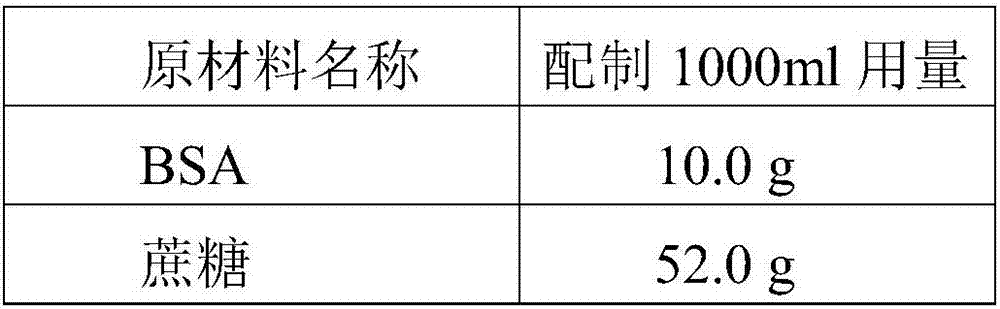
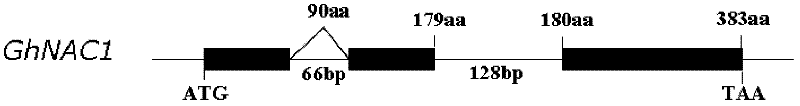Patents
Literature
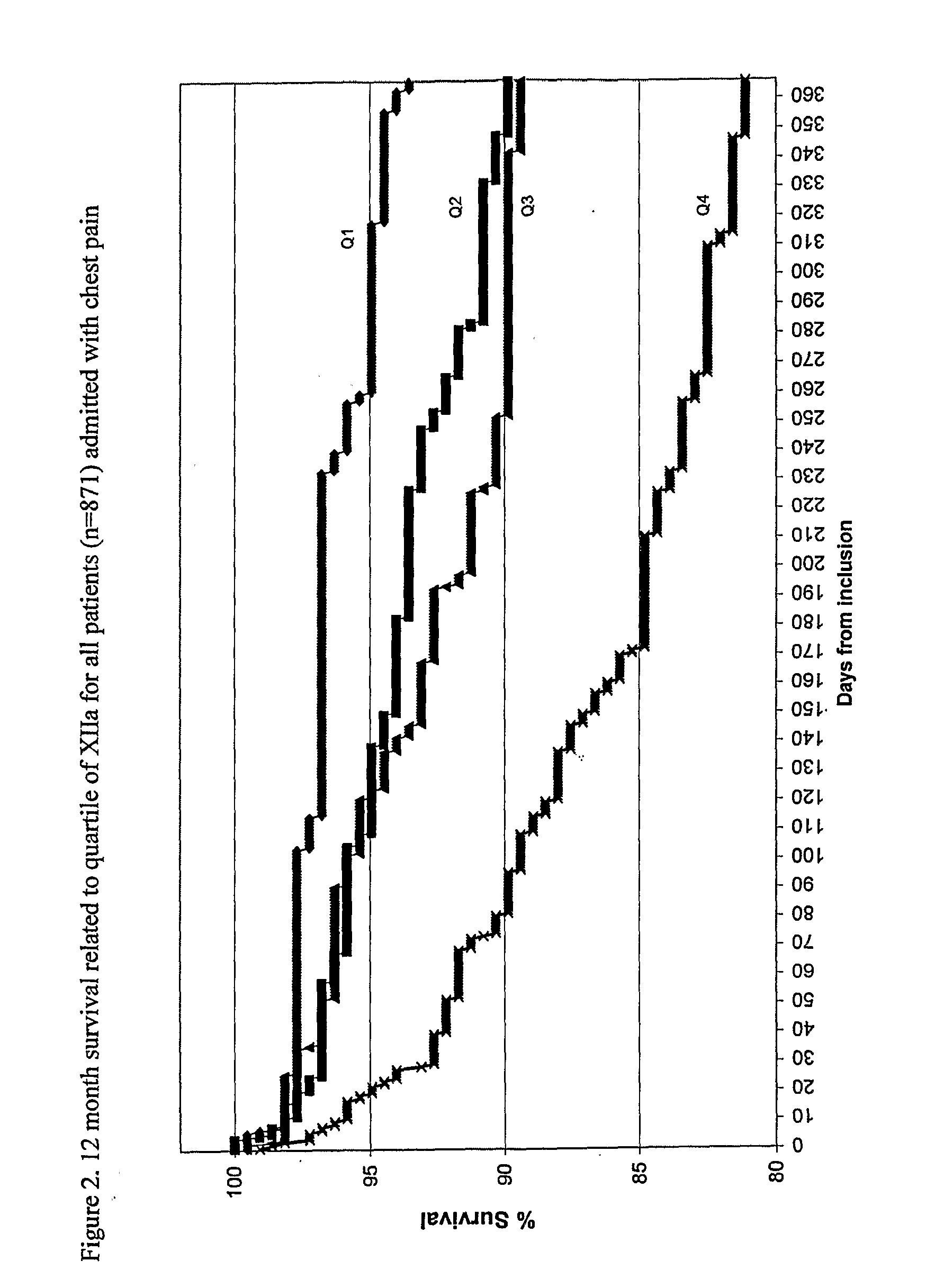
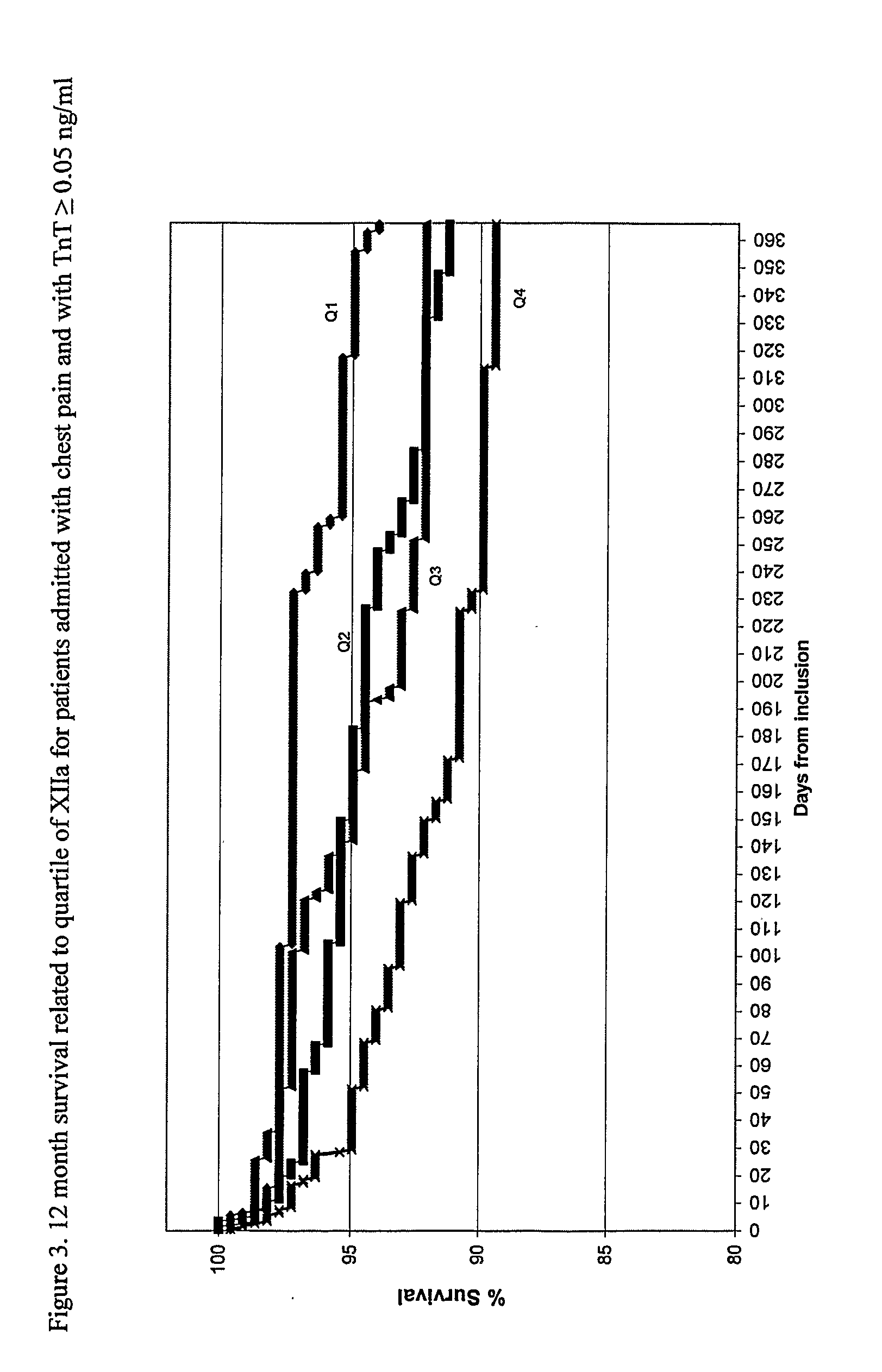
55 results about "Activate factor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Activated Factor X (FXa) Factor X (FX), as known as Stuart-Prower factor or prothrombinase, is the first member of the final common pathway (thrombin pathway) in the coagulation cascade. Activated by factor IXa or factor VIIa, Factor X turn into it's activated form, factor Xa (FXa), which is capable of activating factor II (prothrombin)...
Bone graft
ActiveUS7163691B2Fast reduction in osteoinductiveGood curative effectOrganic active ingredientsImpression capsOSTEOINDUCTIVE FACTORIn vivo
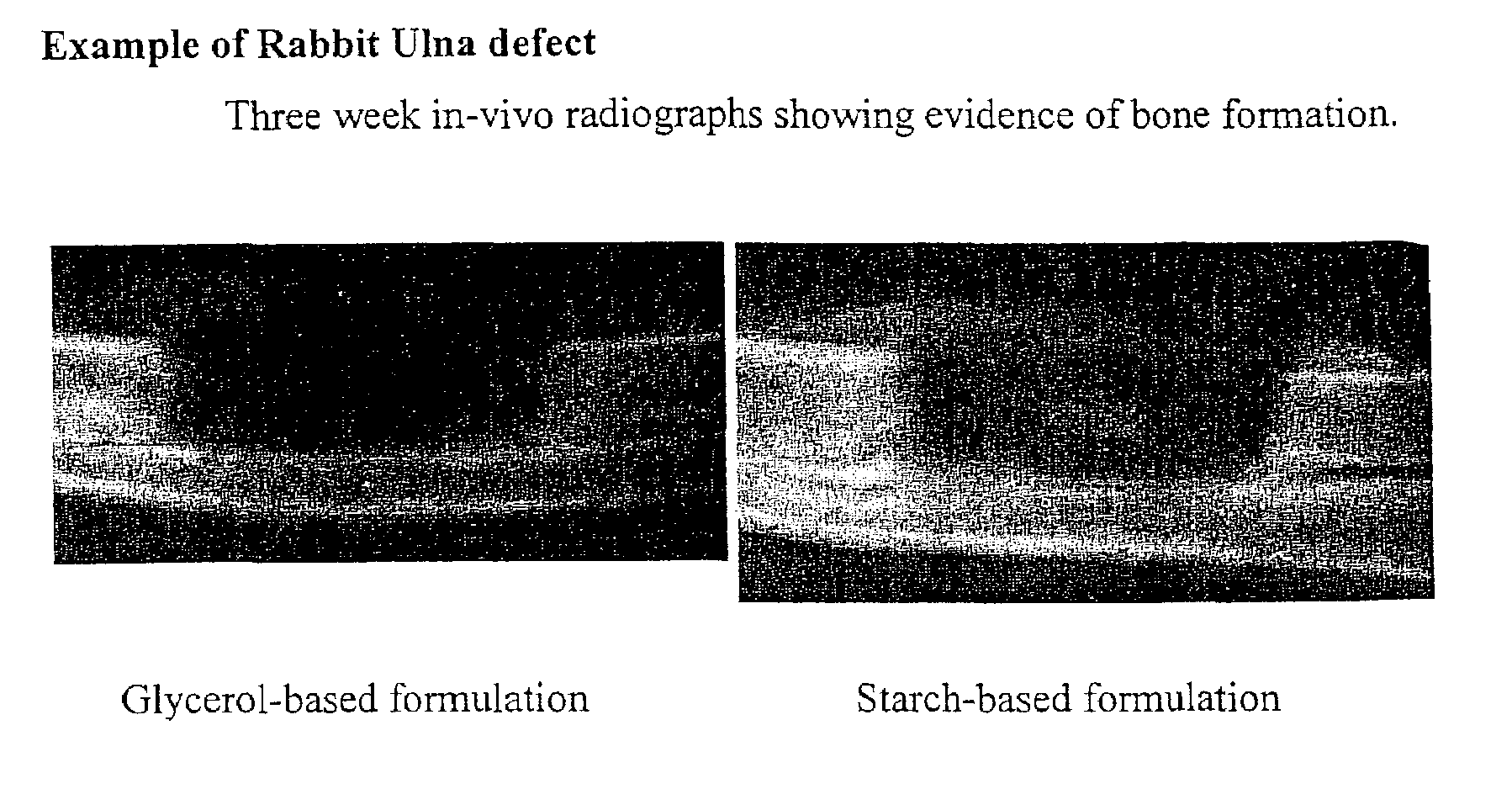
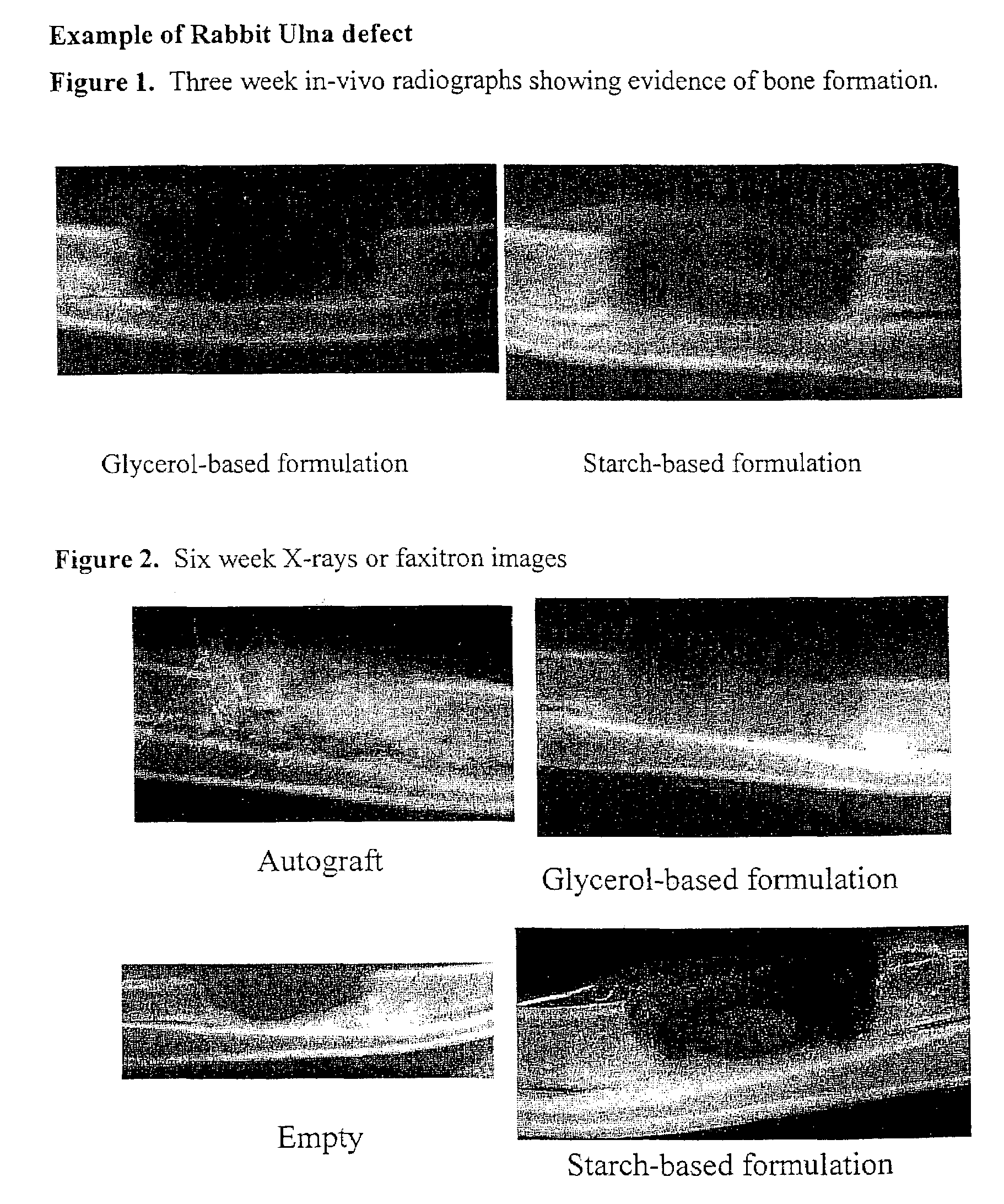
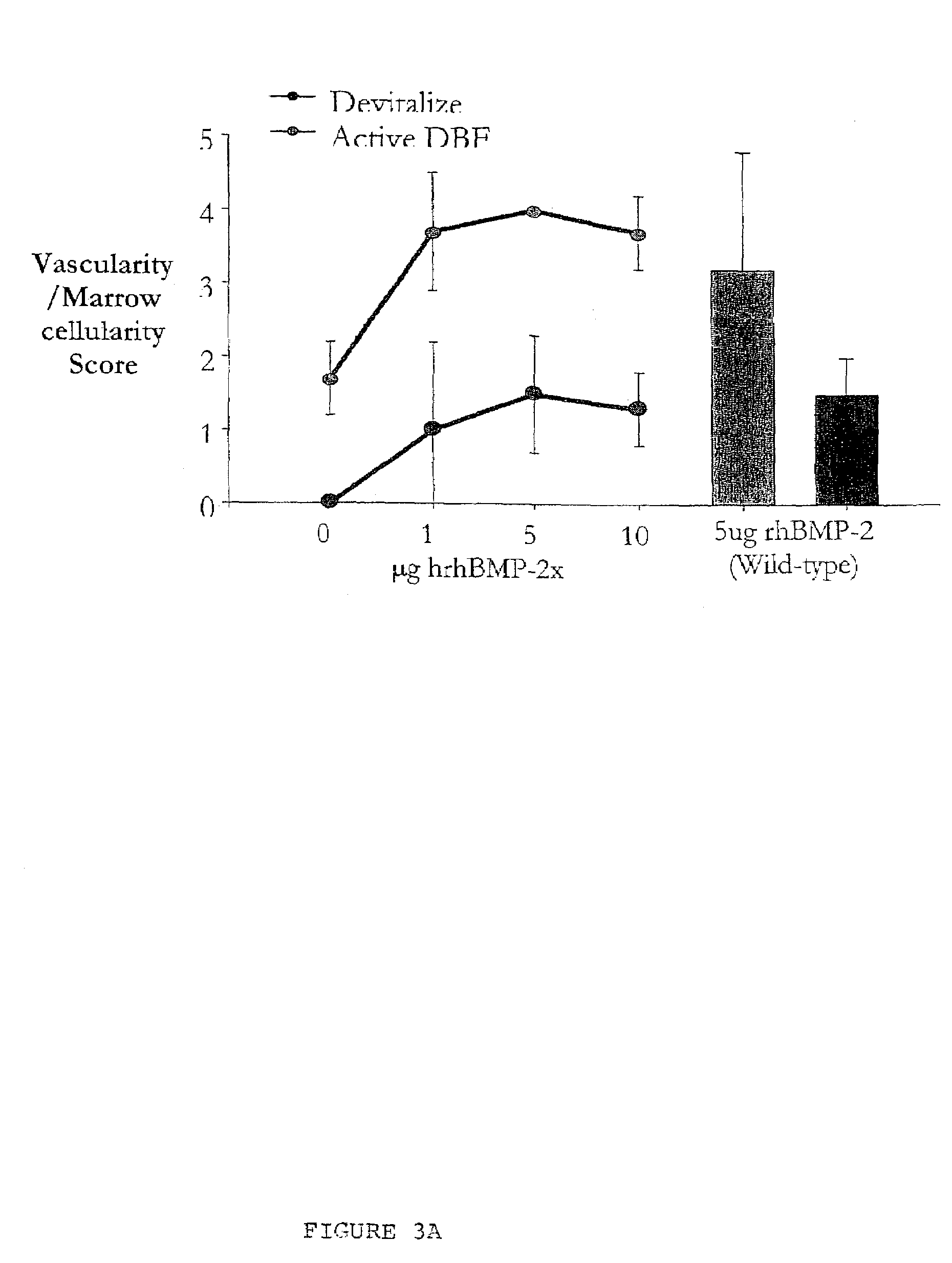
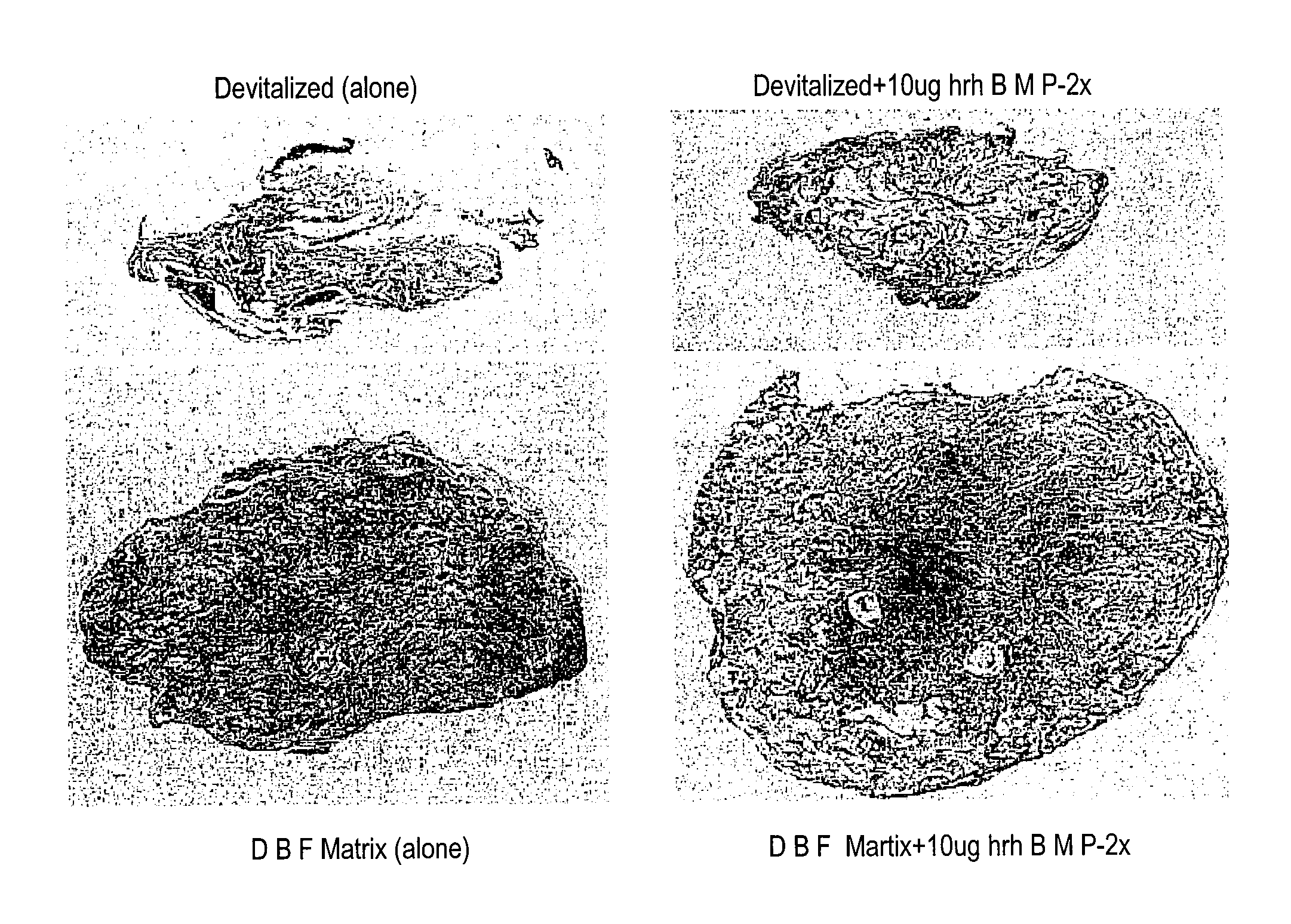
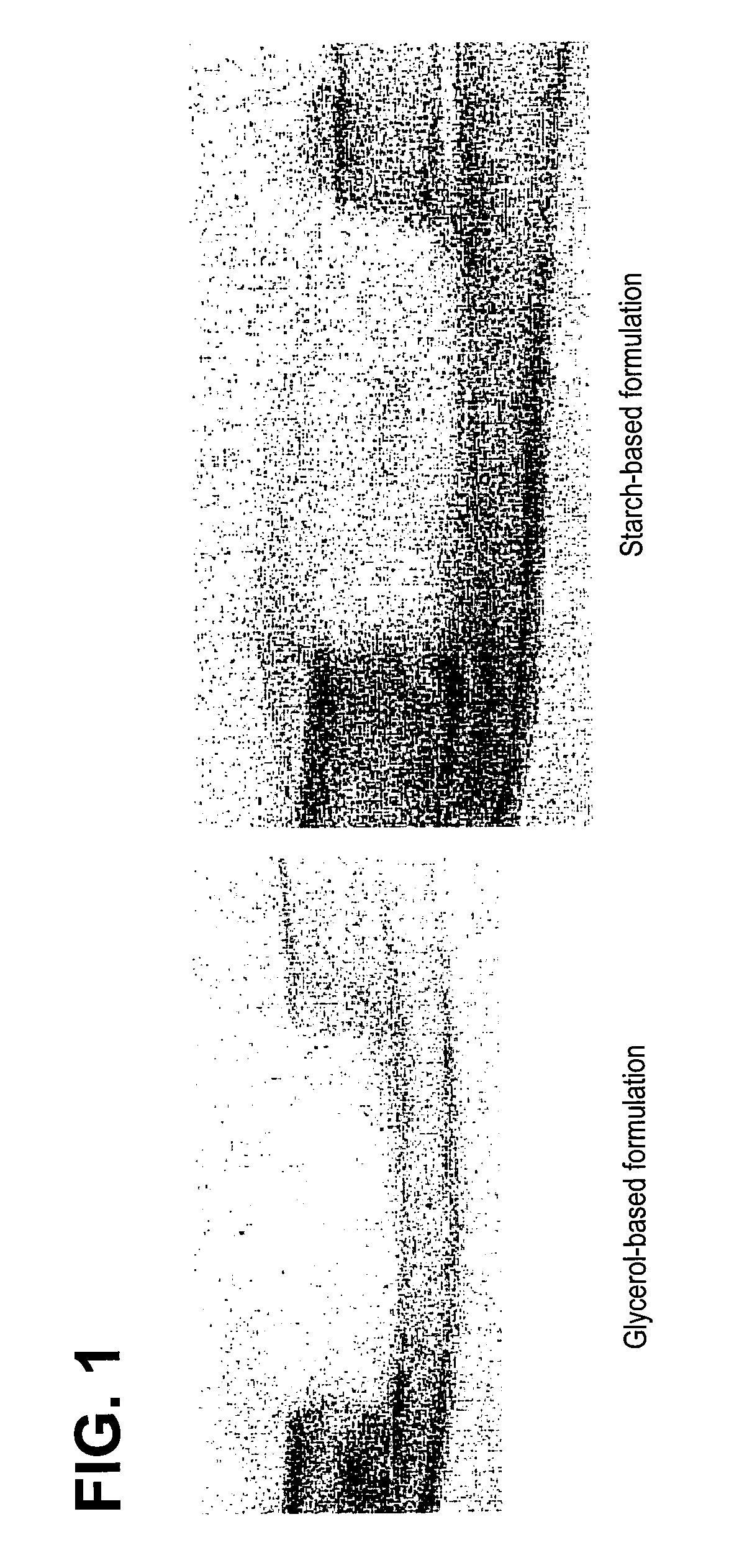
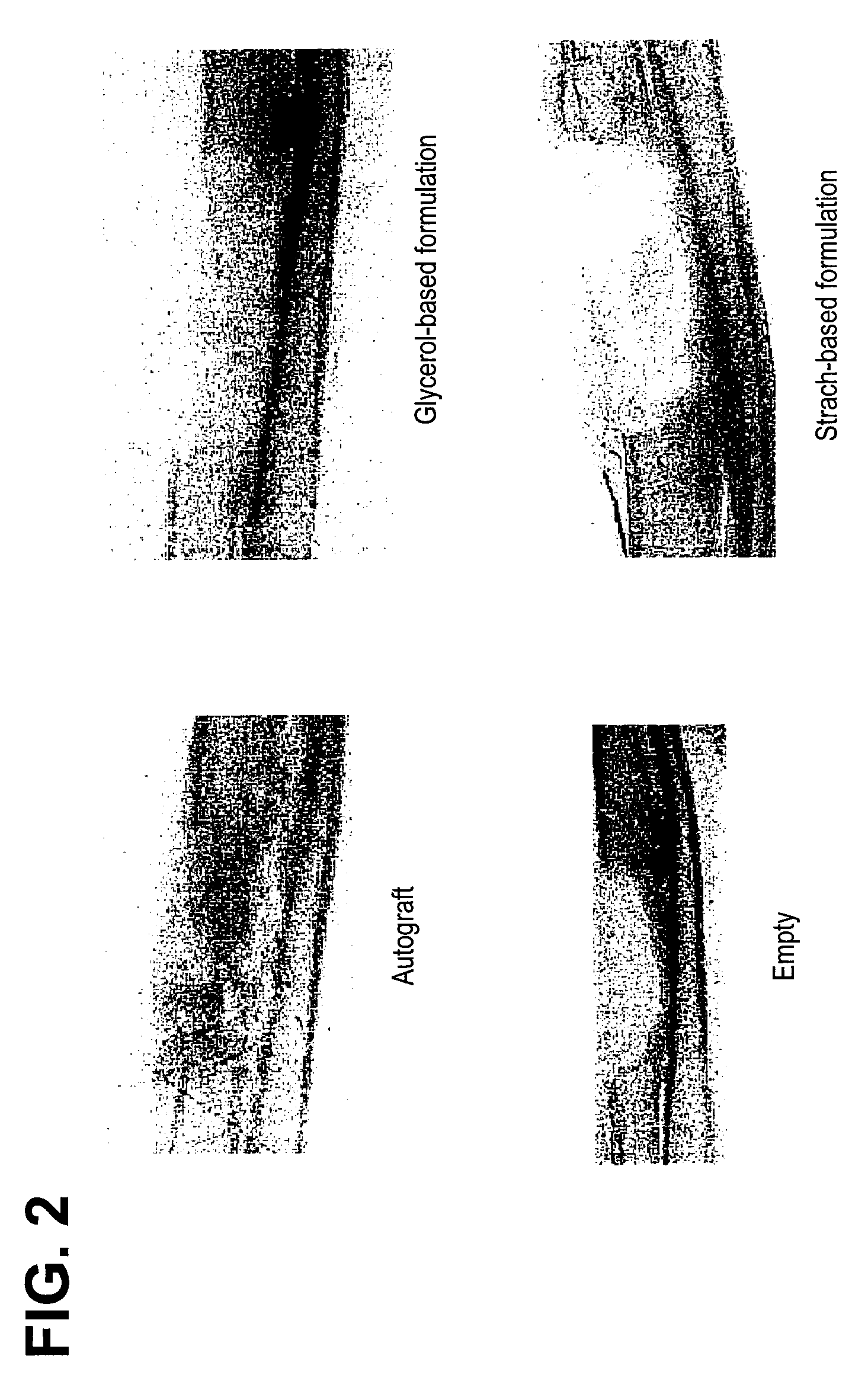
An improved demineralized bone matrix (DBM) or other matrix composition is provided that has been mixed with a stabilizing agent that acts as (1) a diffusion barrier, (2) a enzyme inhibitor, (3) a competitive substrate, or (4) a masking moiety. A diffusion barrier acts as a barrier so as to protect the osteoinductive factors found in DBM from being degraded by proteolytic and glycolytic enzymes at the implantation site. Stabilizing agents may be any biodegradable material such as starches, modified starches, cellulose, dextran, polymers, proteins, and collagen. As the stabilizing agents degrades or dissolves in vivo, the osteoinductive factors such as TGF-β, BMP, and IGF are activated or exposed, and the activated factors work to recruit cells from the preivascular space to the site of injury and to cause differentiation into bone-forming cells. The invention also provides methods of preparing, testing, and using the inventive improved osteodinductive matrix compositions.
Owner:WARSAW ORTHOPEDIC INC
Bone Graft
ActiveUS20080145392A1Good curative effectSimple compositionAdditive manufacturing apparatusBone implantOSTEOINDUCTIVE FACTORIn vivo
An improved demineralized bone matrix (DBM) or other matrix composition is provided that has been mixed with a stabilizing agent that acts as (1) a diffusion barrier, (2) a enzyme inhibitor, (3) a competitive substrate, or (4) a masking moiety. A diffusion barrier acts as a barrier so as to protect the osteoinductive factors found in DBM from being degraded by proteolytic and glycolytic enzymes at the implantation site. Stabilizing agents may be any biodegradable material such as starches, modified starches, cellulose, dextran, polymers, proteins, and collagen. As the stabilizing agents degrades or dissolves in vivo, the osteoinductive factors such as TGF-.beta., BMP, and IGF are activated or exposed, and the activated factors work to recruit cells from the preivascular space to the site of injury and to cause differentiation into bone-forming cells. The invention also provides methods of preparing, testing, and using the inventive improved osteodinductive matrix compositions
Owner:WARSAW ORTHOPEDIC INC
Therapeutic use of cis-element decoys in vivo
The invention provides for the use of oligodeoxynucleotide decoys for the prophylactic or therapeutic treatment of diseases associated with the binding of endogenous transcription factors to genes involved in cell growth, differentiation and signalling or to viral genes. By inhibiting endogenous trans-activating factors from binding transcription regulatory regions, the decoys modulate gene expression and thereby regulating pathological processes including inflammation, intimal hyperplasia, angiogenesis, neoplasia, immune responses and viral infection. The decoys are administered in amounts and under conditions whereby binding of the endogenous transcription factor to the endogenous gene is effectively competitively inhibited without significant host toxicity. The subject compositions comprise the decoy molecules in a context which provides for pharmacokinetics sufficient for effective therapeutic use.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
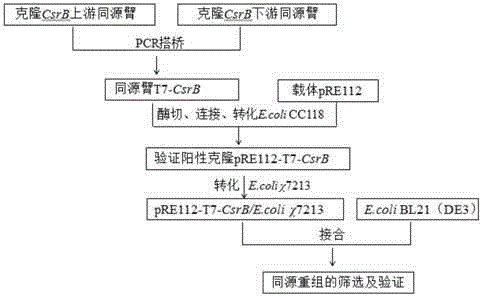
Genetic engineering bacterium for high-yield phloroglucinol as well as construction method and application of genetic engineering bacterium
ActiveCN104388371AIncrease productionImprove synthesis abilityBacteriaPeptidesCarbon metabolismCarbon storage
The invention discloses a genetic engineering bacterium for high-yield phloroglucinol as well as a construction method and an application of the genetic engineering bacterium and belongs to the technical field of genetic engineering. The genetic engineering bacterium over-expresses carbon storage and regulation factor gene CsrB shown by SEQ ID NO.1 and co-expresses a polyketide synthase gene phlD, a multiple resistance activating factor gene marA and acetyl CoA carboxylase gene ACCase. The invention also provides the construction method and the application of the genetic engineering bacterium for the high-yield phloroglucinol. With the adoption of the genetic engineering bacterium, the yield of the phloroglucinol is firstly increased by 115.6 percent by adopting a mode of globally regulating carbon metabolism after post-transcriptional level of the genetic engineering bacterium, and the genetic engineering bacterium has a high industrial application value.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
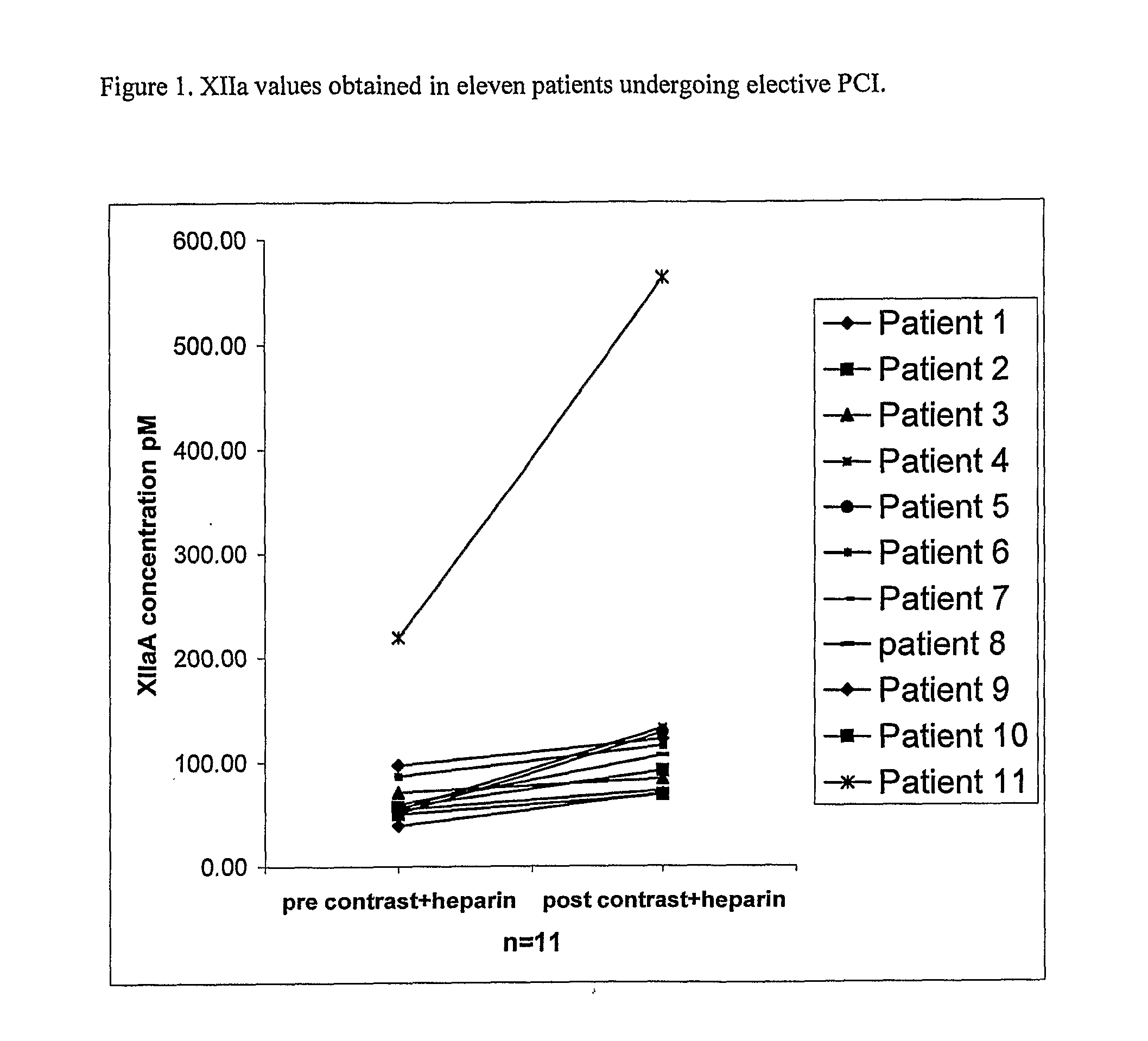
Anti-Factor Xlla Therapy
InactiveUS20090304685A1Immunoglobulins against blood coagulation factorsMicrobiological testing/measurementActivate factorAntibody
A method is disclosed for preventing arterial thrombosis in a subject comprising administering to said subject a therapeutically effective amount of an antibody or epitope-binding fragment or derivative thereof, wherein said antibody, fragment or derivative specifically binds to activated Factor XIIa and prevents the interaction of activated Factor XIIa with its physiological substrates.
Owner:AXIS SHIELD DIAGNOSTICS
Production technique and application of follicular cell activator-containing anti-hair loss restoring preparation
InactiveCN109908329ARegulation of proliferationRegulate differentiationCosmetic preparationsHair cosmeticsWnt3A ProteinMicrocirculation
The invention relates to a follicular cell activator composition and preparation and their application. A follicular cell activator comprises at least two of a bFGF (basic fibroblast growth factor), an KGF or KGF1 (keratinocyte growth factor or keratinocyte growth factor 1), a KGF2 (keratinocyte growth factor 2), an IGF-1 (insulin-like growth factor 1), a VEGF (vascular endothelial growth factor),an HGF or HGF alpha (hepatocyte growth factor or hepatocyte growth factor alpha), an FGF18 (fibroblast growth factor 18), an SCF (stem cell factor), an FGF20 (fibroblast growth factor 20), an NGF (never growth factor), a GH (growth hormone) and Wnt3a protein. The follicular cell activator preparation herein can regulate proliferation, differentiation, regeneration and metabolism of follicular cells, and improve microcirculation to restore follicles and effectively treat seborrheic alopecia and other types of hair loss.
Owner:北京中农创新生物工程研究院有限公司
Gene modification method for increasing yield of phloroglucinol and application of same
InactiveCN104388457AIncrease productionHigh industrial application valueBacteriaMicroorganism based processesBiotechnologyCarbon metabolism
The invention discloses a gene modification method for increasing the yield of phloroglucinol and application of the same, and belongs to the technical field of genetic engineering. The gene modification method comprises the following steps: obtaining a mutant strain by virtue of a mode of knocking out or inserting a global regulating factor arcA gene of an original strain, converting the state of the mutant strain to a competent state, introducing recombinant plasmids containing a polyketide synthase gene phlD, a multiple resistance activating factor marA and acetyl CoA carboxylase gene ACCase, and obtaining a recombinant cell. The invention also provides a method of producing the phloroglucinol by utilizing the recombinant cell. According to the gene modification method and application disclosed by the invention, the yield of the phloroglucinol after fermentation is firstly increased by 2.06 times by adopting a mode of globally regulating carbon metabolism after post-transcriptional level, and the gene modification method and application have high industrial application value.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
Depression gene
InactiveUS7410944B1Avoid depressionImprove disease symptomsPeptide/protein ingredientsDepsipeptidesGermline mutationGenetics human
Owner:MYRIAD GENETICS
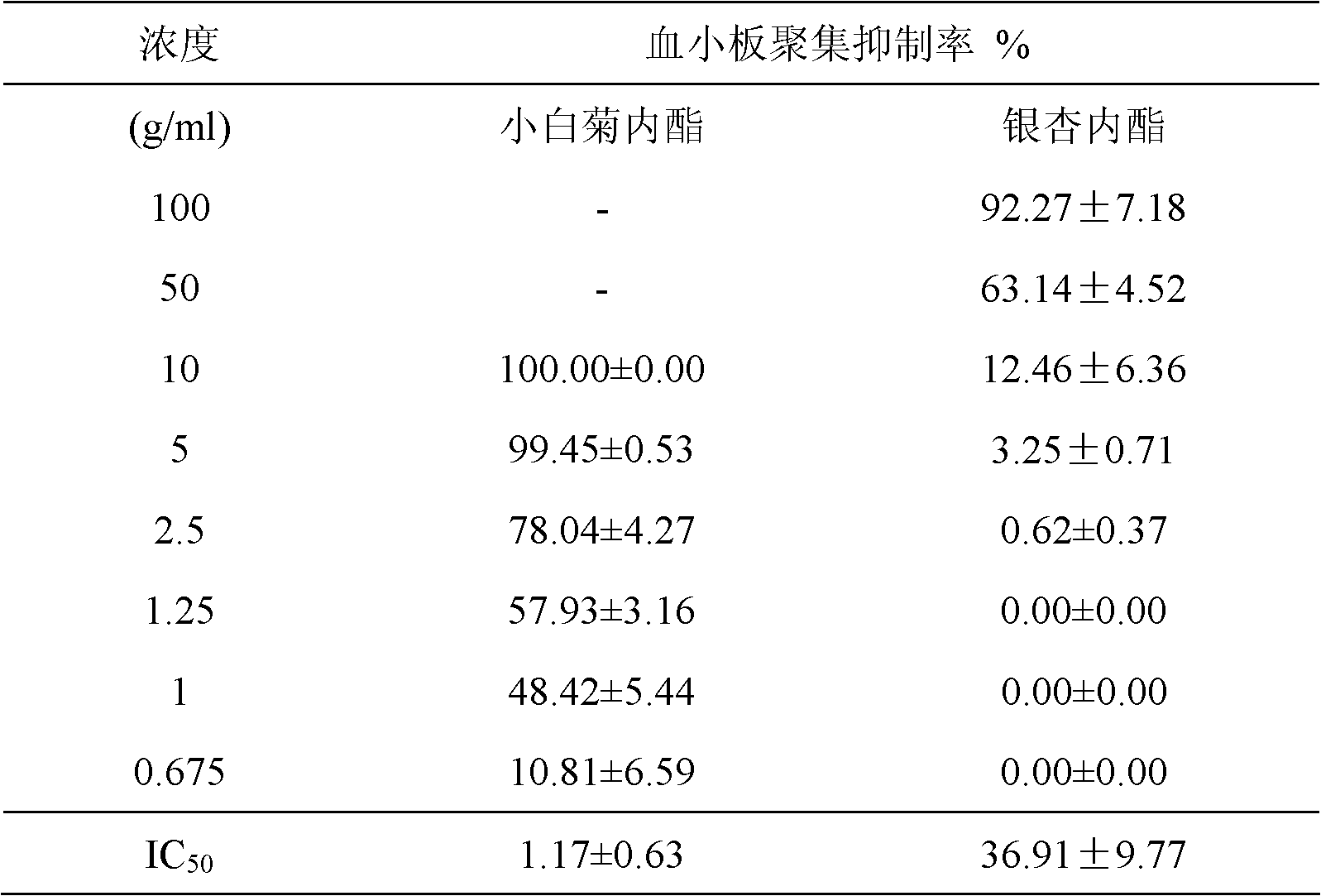
Application of parthenolide as platelet-activating factor (PAF) antagonist
InactiveCN102579426AReduce contentAntibacterial agentsOrganic active ingredientsThrombusHepatic fibrosis
The invention discloses application of parthenolide as a platelet-activating factor (PAF) inhibitor. The parthenolide can be used as a safe PAF antagonist to be applied to treatment, auxiliary treatment and prevention of diseases such as thrombus, atherosclerosis, ischemic cardio-vascular disease, cerebral ischemia, acute pancreatitis, endotoxic shock, asthma, hepatic fibrosis and hepatocirrhosis, injury of nerve, gastrointestinal ulcer and necrosis, psoriasis, systemic lupus erythematosus and acquired immune deficiency syndrome related to platelet-activating factor.
Owner:韩颖
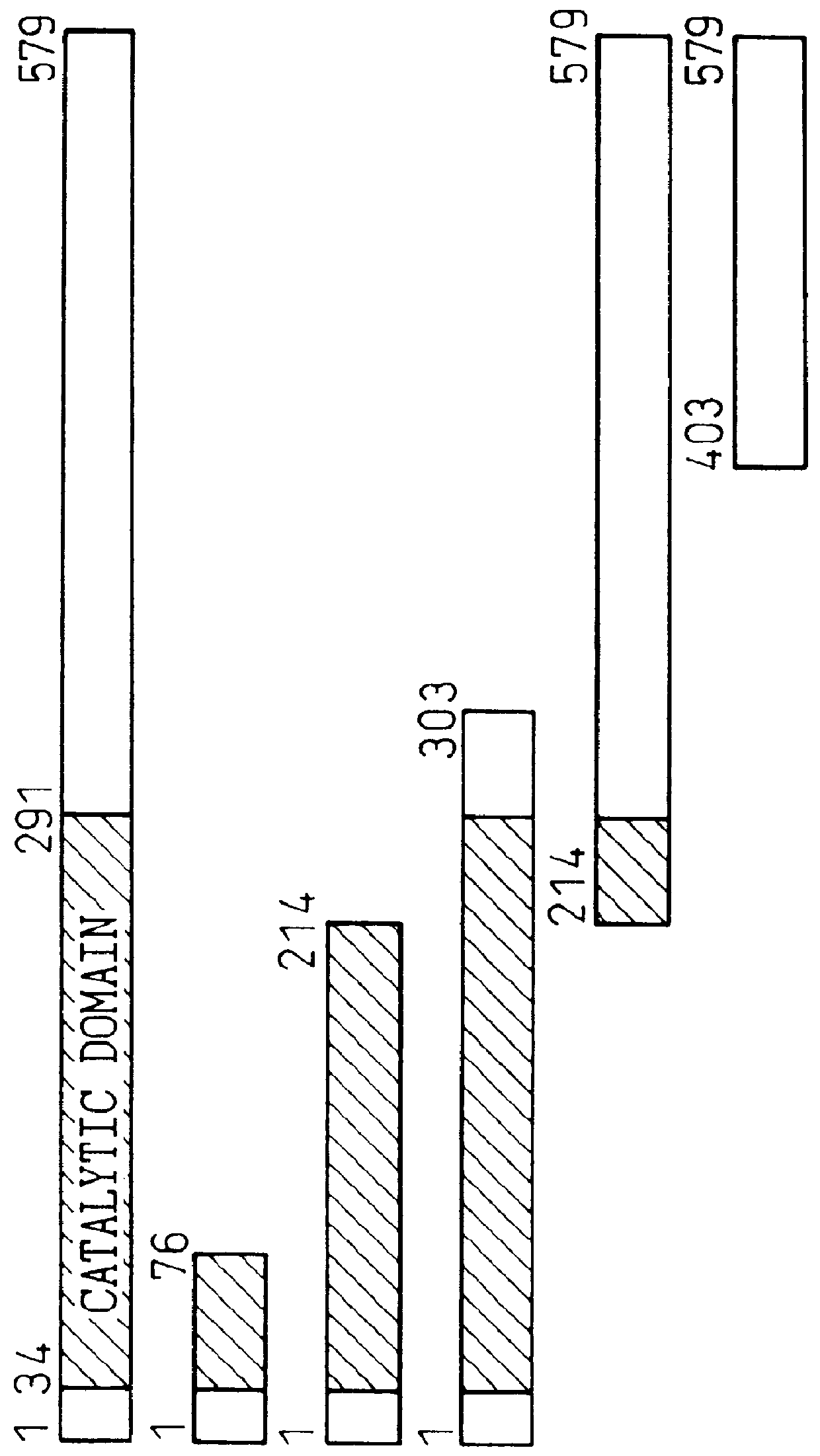
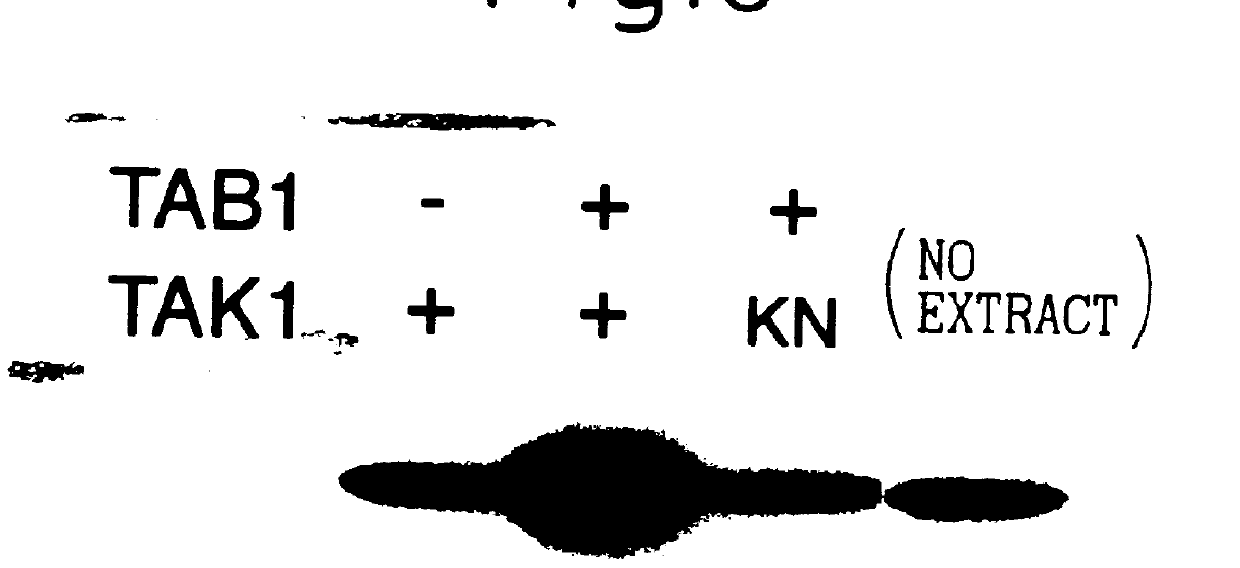
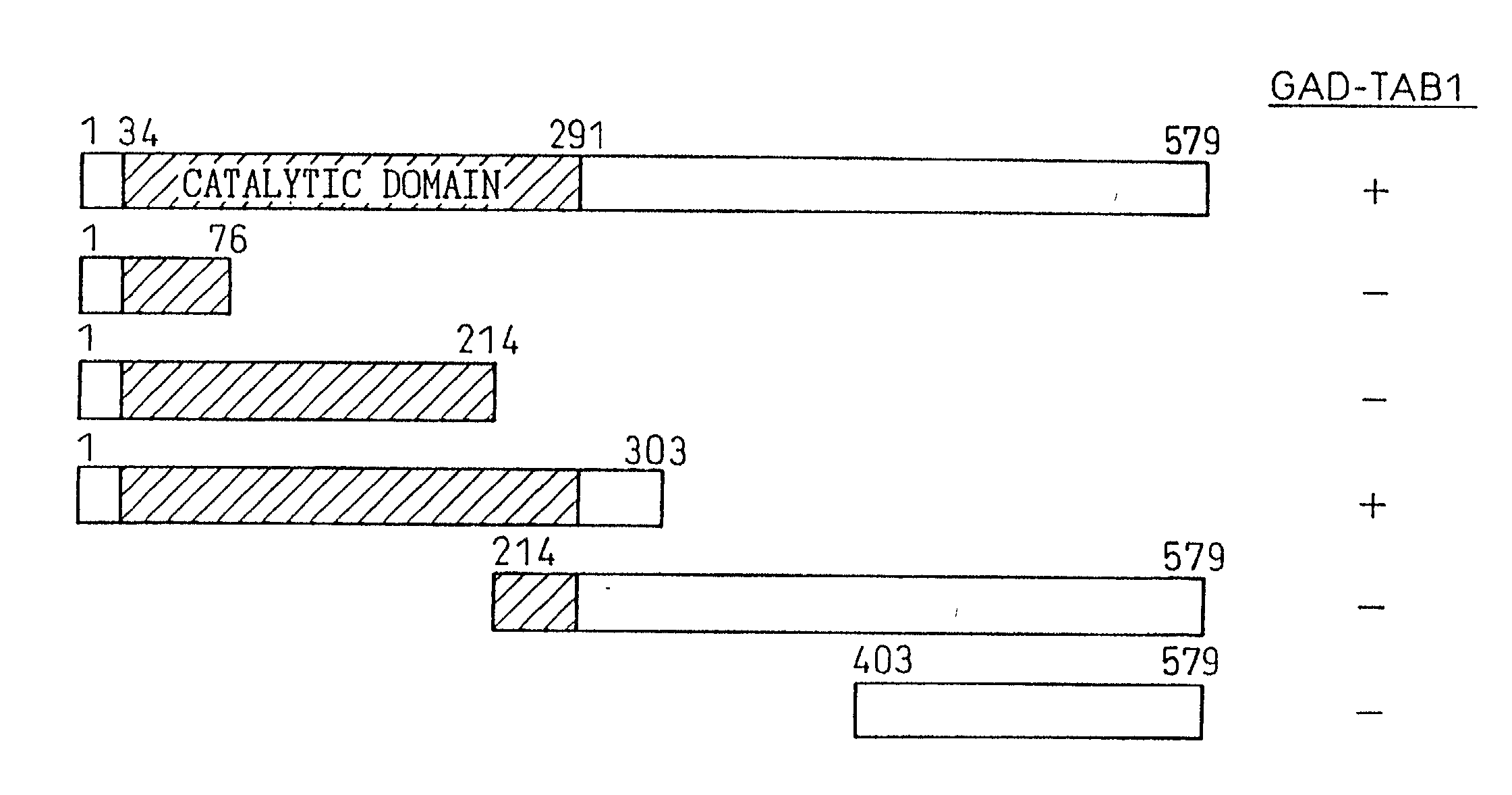
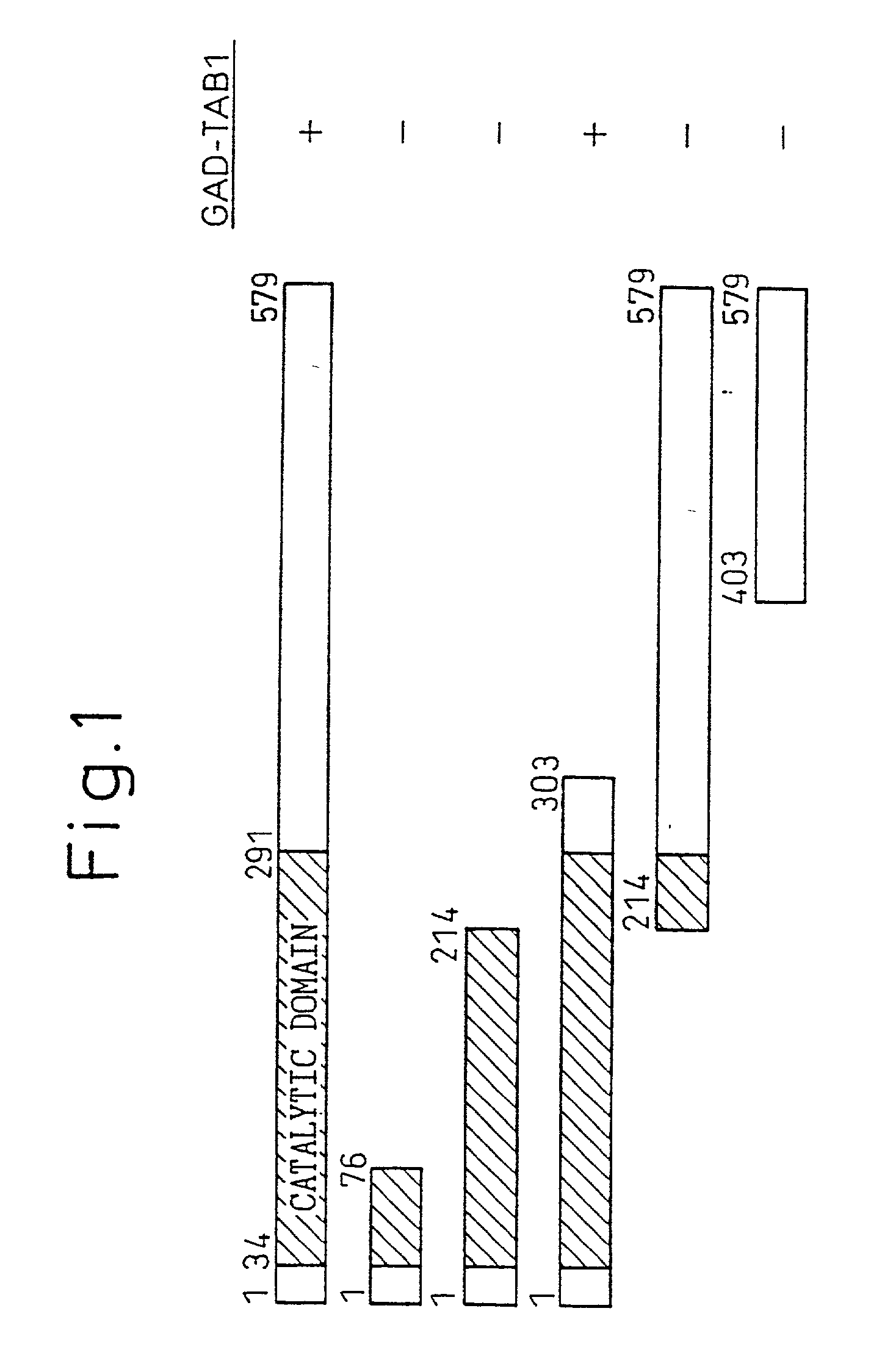
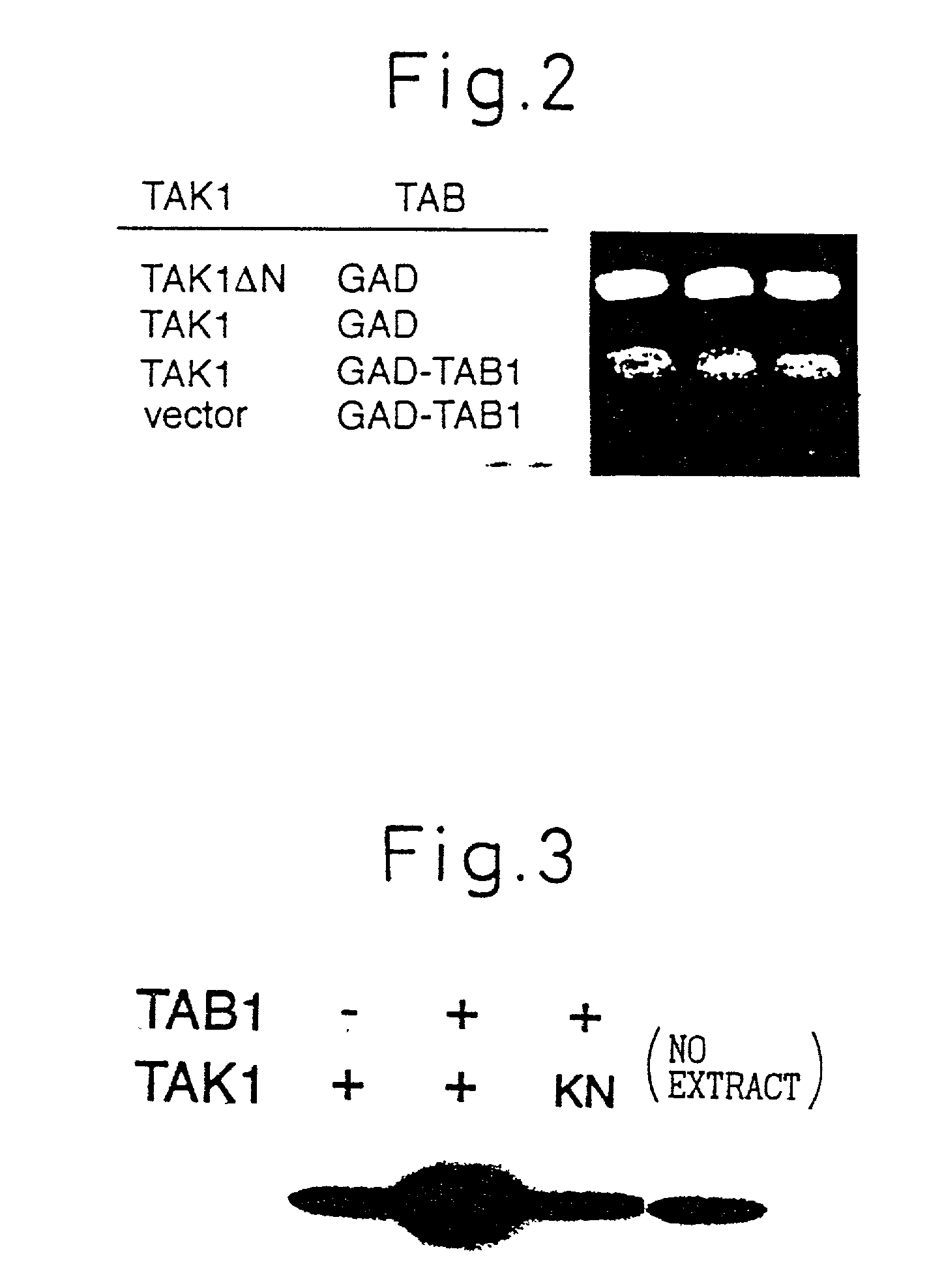
TAB1 protein and DNA coding therefore
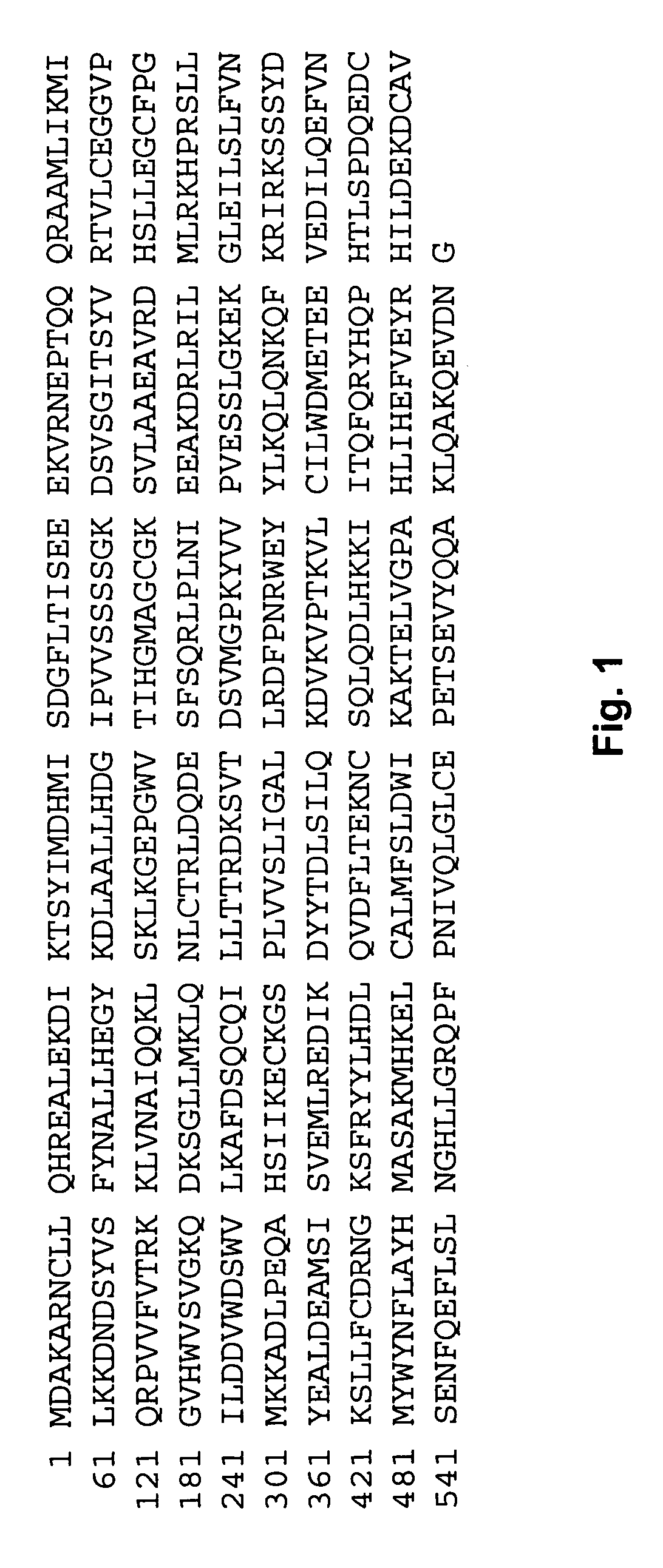
TAB1 protein having activity which activates factor TAK1 in the TGF- beta signaling pathway, and having the amino acid sequence shown in FIG. 1.
Owner:CHUGAI PHARMA CO LTD
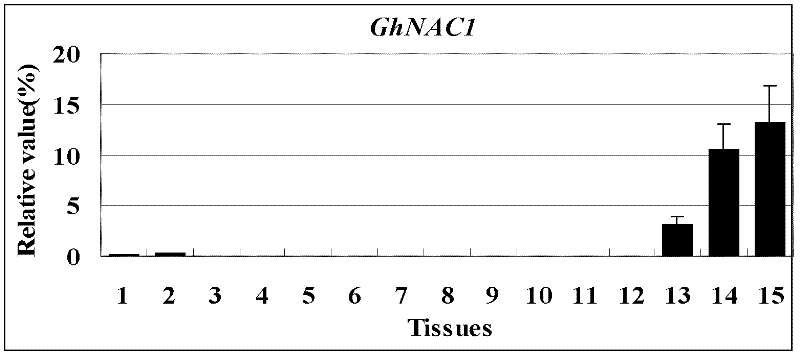
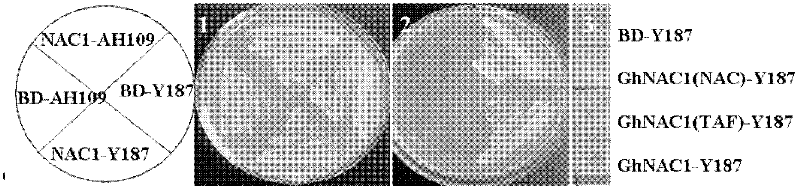
Cloning and identification of cotton fiber cell secondary wall development-associated GhNAC1 gene
InactiveCN102399792ATranscriptional self-activatingRegulates secondary wall developmentPlant peptidesFermentationExonCell wall
The invention discloses a novel cotton fiber secondary development specific expression gene GhNAC1 full-length sequence, and relates to an important cotton fiber development-associated regulator gene element. The GhNAC1 gene contains three exons and two introns, and is coded with an NAC (N Acetyl L Cysteine) transcription factor. A large quantity of mRNAs (messenger Ribonucleic Acids) of the gene are accumulated specifically at the secondary development stage of the cotton fiber cells, which indicates that the gene is a fiber secondary wall development specific gene. GhNAC1 protein is positioned in a cell nucleolus and has the capability of activating transcription independently, which indicates that the protein serving as a transcription activating factor plays a role in cotton fiber development. GhNAC1 is expressed excessively in arabidopsis, so that leaves crimp upwards, cell walls are thickened, ectopic sedimentation of lignin and the like is detected in foliar epidermic cells, so that the hemicellulose content of foliar cells is greatly increased. As proved by the results, the GhNAC1 can be used for regulating the biosynthesis of cell secondary walls, and plays a key role in the developing process of cotton fiber.
Owner:HUAZHONG NORMAL UNIV
TAB1 protein and DNA coding therefore
TAB1 protein having activity which activates factor TAK1 in the TGF-beta signaling pathway, and having the amino acid sequence shown in FIG. 1.
Owner:CHUGAI PHARMA CO LTD
Fibrinolysin of fermented soya beans and culture method thereof
PendingCN106978411AThrombolytic properties are stableLittle effect on proteinMicroorganism based processesPeptidasesBacillus amyloliquefaciensFibrinogen
The invention relates to fibrinolysin of fermented soya beans and a culture method thereof. The fibrinolysin of fermented soya beans is prepared through fermentation, separation and purification on a strain Jxnuwx-1 as a culture strain, wherein the preservation number of the strain in the fermented soya beans is CCTCC M2014638 and the taxonomic name of the strain is Bacillus amyloliquefaciens. The fibrinolysin of fermented soya beans is fibrinolysin, the relative molecular mass of which is 29kDa. The optimum action temperature is 41 DEG C, the temperature stabilizing range is 23-43 DEG C, the optimum pH is 7.6, and the pH stabilizing interval is 7.0-11.0. The fibrinolysin of fermented soya bean can successively degrade Aalpha, Bbeta and Cgamma chains of human fibrin (proteinogen) within 4h. The fibrinolysin of fermented soya beans not only can degrade fibrous protein and fibrinogen, but also can serve as a plasminogen activating factor to degrade thrombin. The influence of the fibrinolysin of fermented soya beans on proteins in a human blood is relatively small, and the fibrinolysin of fermented soya beans provides a novel path for development and utilization of antithrombotic drugs.
Owner:JIANGXI NORMAL UNIV
Therapeutic use of cis-element decoys in vivo
InactiveUS20020128217A1Inhibit bindingBiocidePeptide/protein ingredientsDiseaseIntimal proliferation
The invention provides for the use of oligodeoxynucleotide decoys for the prophylactic or therapeutic treatment of diseases associated with the binding of endogenous transcription factors to genes involved in cell growth, differentiation and signaling or to viral genes. By inhibiting endogenous trans-activating factors from binding transcription regulatory regions, the decoys modulate gene expression and thereby regulating pathological processes including inflammation, intimal hyperplasia, angiogenesis, neoplasia, immune responses and viral infection. The decoys are administered in amounts and under conditions whereby binding of the endogenous transcription factor to the endogenous gene is effectively competitively inhibited without significant host toxicity. The subject compositions comprise the decoy molecules in a context which provides for pharmacokinetics sufficient for effective therapeutic use.
Owner:DZAU VICTOR J +2
Methods and compositions for the treatment, prevention, and alleviation of bone and cartilage diseases or injuries and hair loss
InactiveUS20120171182A1Treating and preventing and alleviating hair lossTreating, preventing, or alleviating hair lossOrganic active ingredientsBiocideDiseaseDexamethasone
The present invention provides pharmaceutical compositions, and methods of preparation and use for the treatment, prevention or alleviation of bone and cartilage diseases or injuries and hair loss. The present invention discloses a method and a pharmaceutical composition comprising mesenchymal stem cells, platelets, activating factors and scaffolding materials for the treatment, prevention, or alleviation of bone diseases, bone injuries or hair loss, and a method and a pharmaceutical composition further comprising dexamethasone for the treatment, prevention, or alleviation of cartilage diseases or injuries.
Owner:PAK JAE WOO
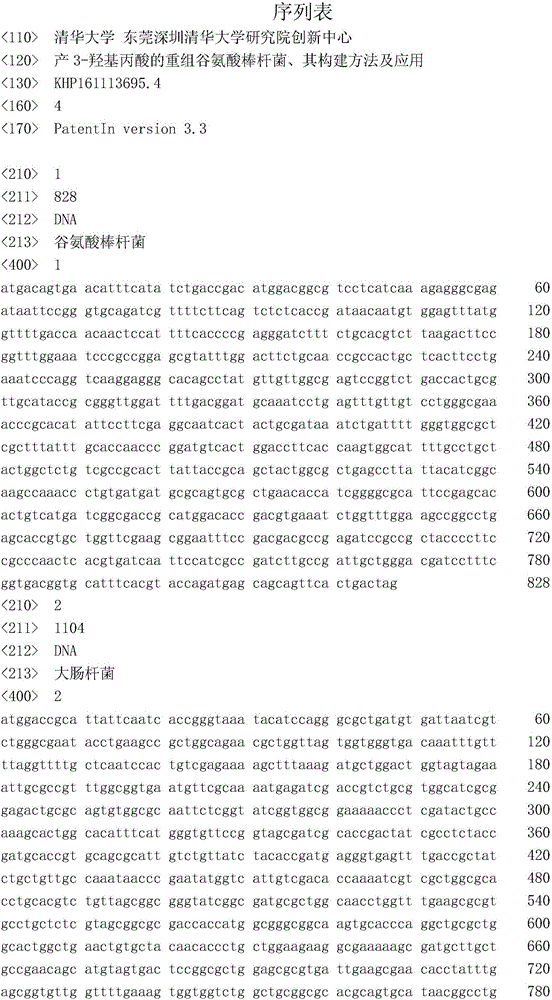
3-hydracrylic-acid-producing recombinant Corynebacterium glutamicum strain, and construction method and application thereof
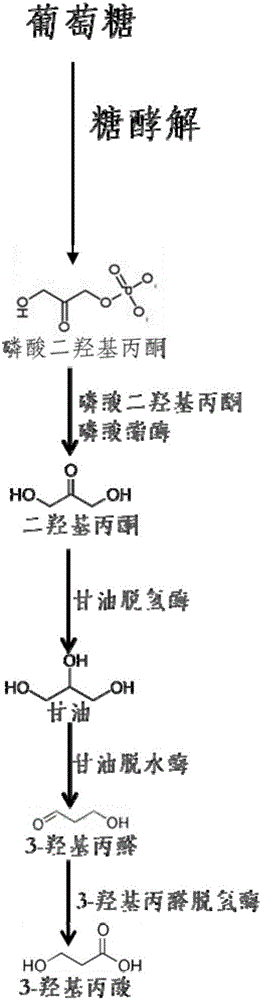
ActiveCN105950529ASolving Tolerance IssuesAddress biosecurityBacteriaMicroorganism based processes3-Hydroxypropionic acidGlycerol
The invention relates to a 3-hydracrylic-acid-producing recombinant Corynebacterium glutamicum strain, and a construction method and application thereof. The construction method of the recombinant Corynebacterium glutamicum strain comprises the following steps: in a Corynebacterium glutamicum strain, overexpressing an endogenous dihydroxyacetone phosphate phosphatase gene hdpA, and overexpressing a glyceroldehydrogenase gene gldA, a glycerol anhydrase and activating factor gene pduCDEGH and a 3-hydroxypropylaldehyde dehydrogenase gene aldH. When the recombinant strain is used for producing 3-hydracrylic acid by fermentation, the Corynebacterium glutamicum strain can perform fermentation by using different cheap raw materials, thereby further lowering the raw material cost. The 3-hydracrylic-acid-producing recombinant Corynebacterium glutamicum strain solves the problems of biosafety and acid tolerance. The strain in the fermentation process can be used in a feed additive as a product. The method can generate fewer byproducts, so that the separation process of the end product 3-hydracrylic acid is simplified.
Owner:GUANGDONG TSINGDA SMART BIOTECH CO LTD
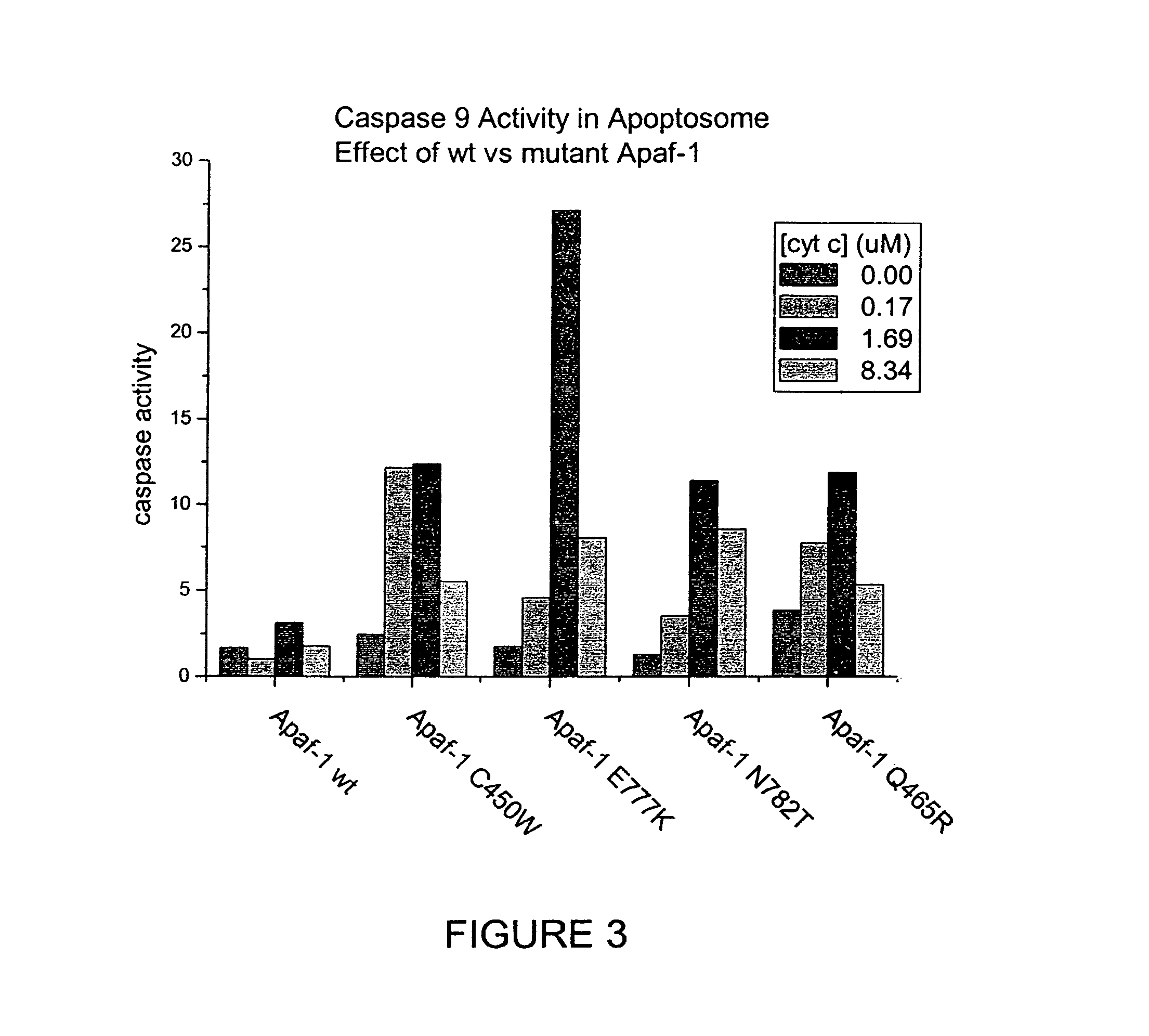
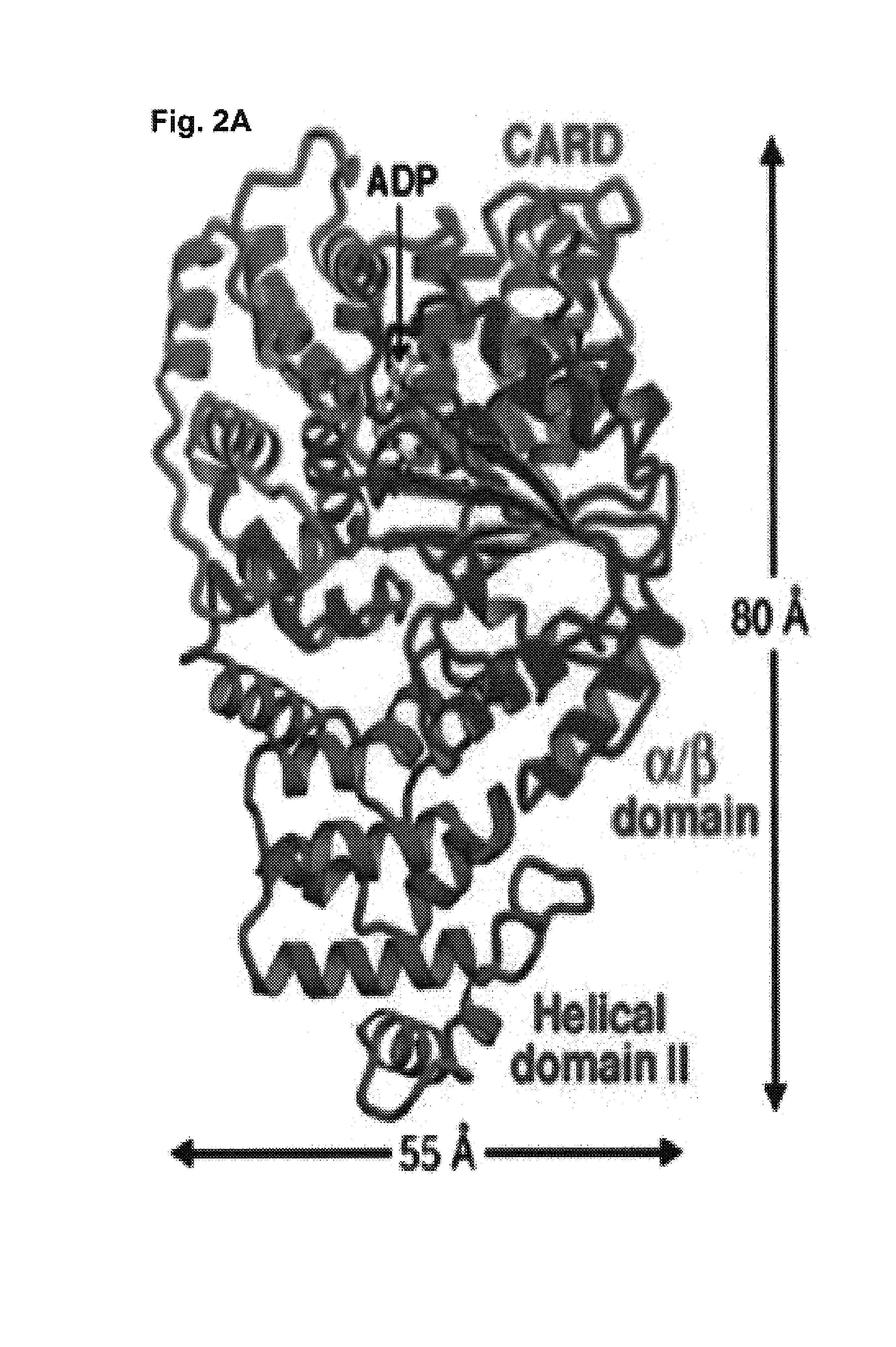
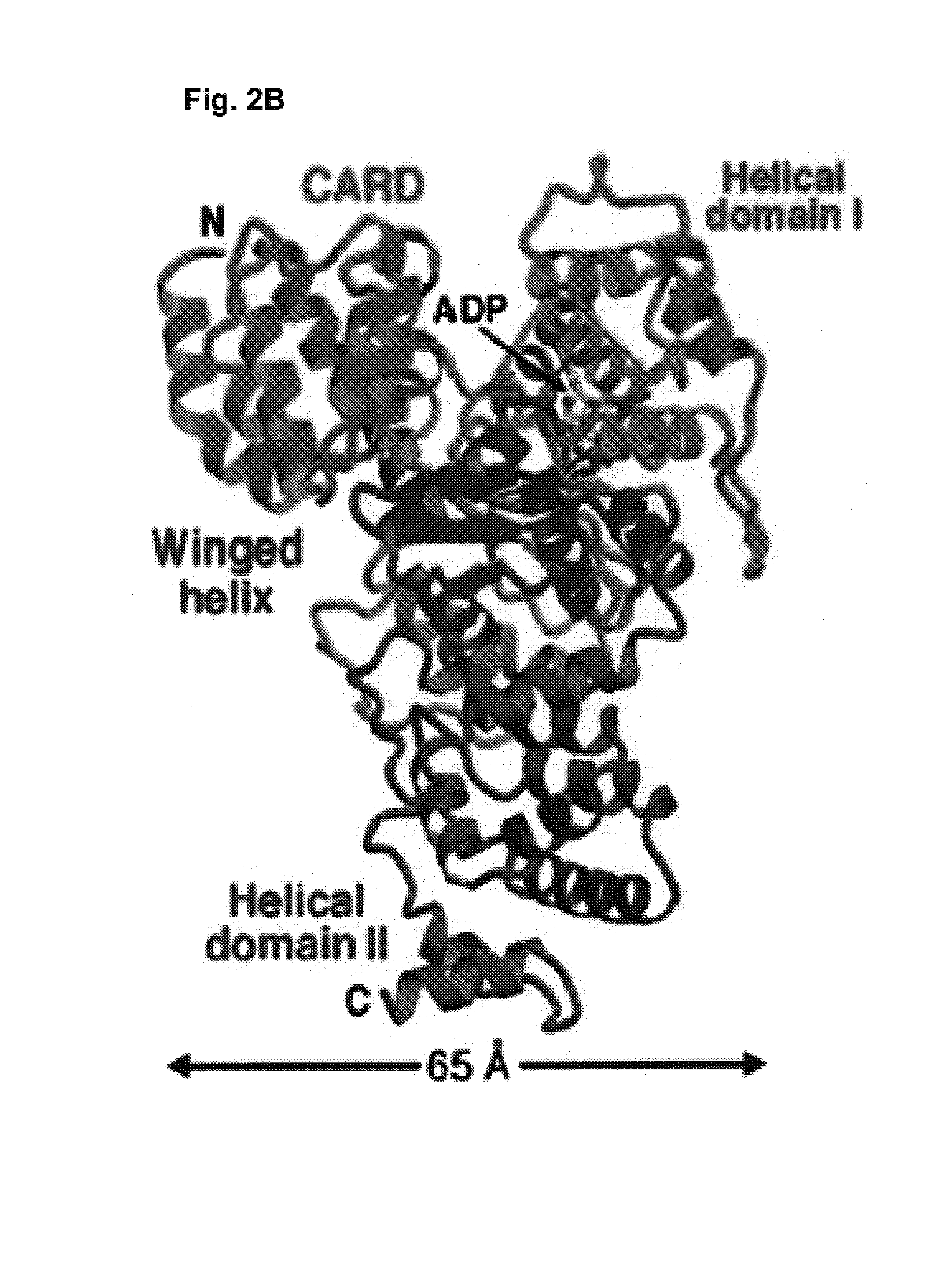
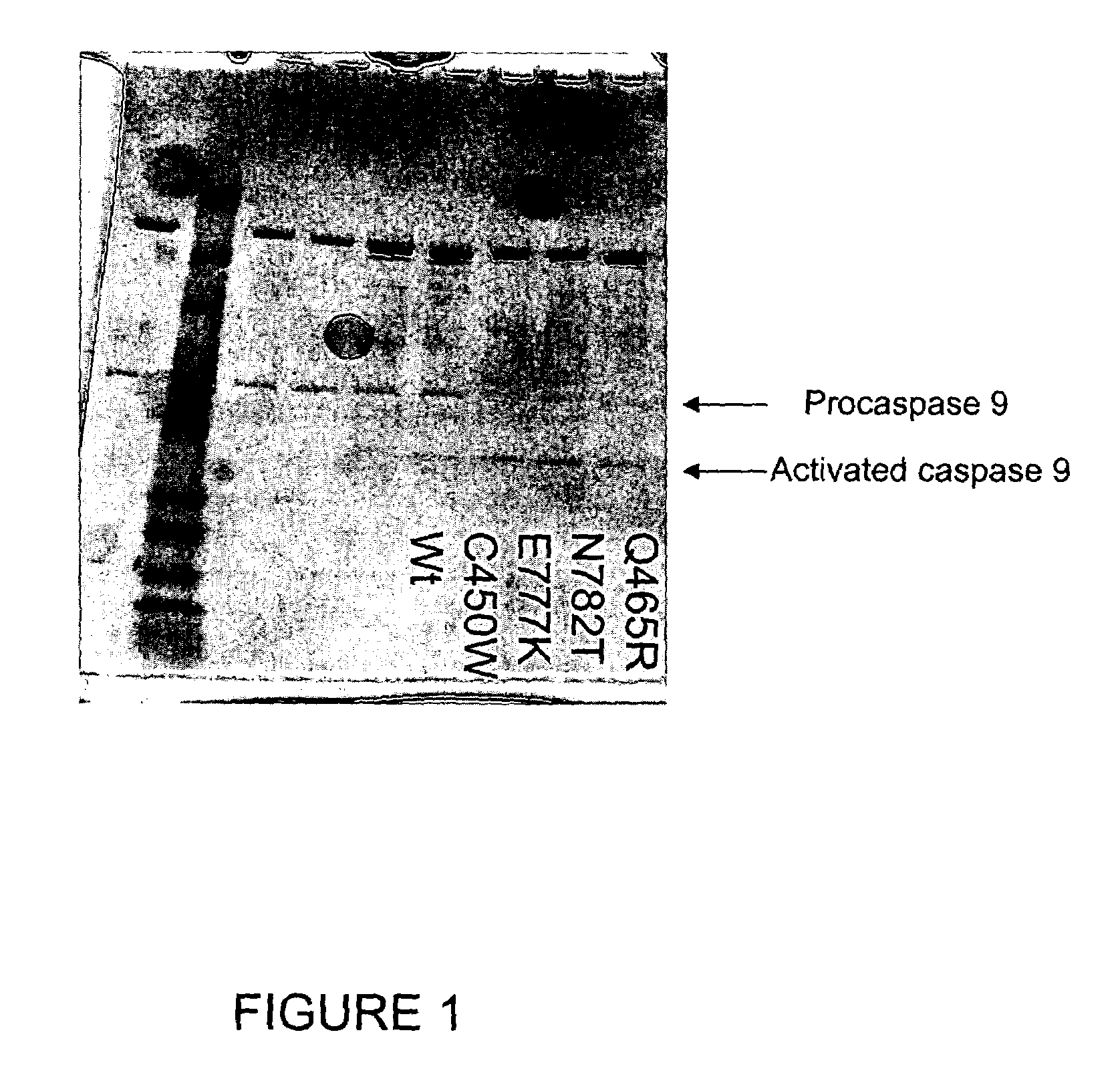
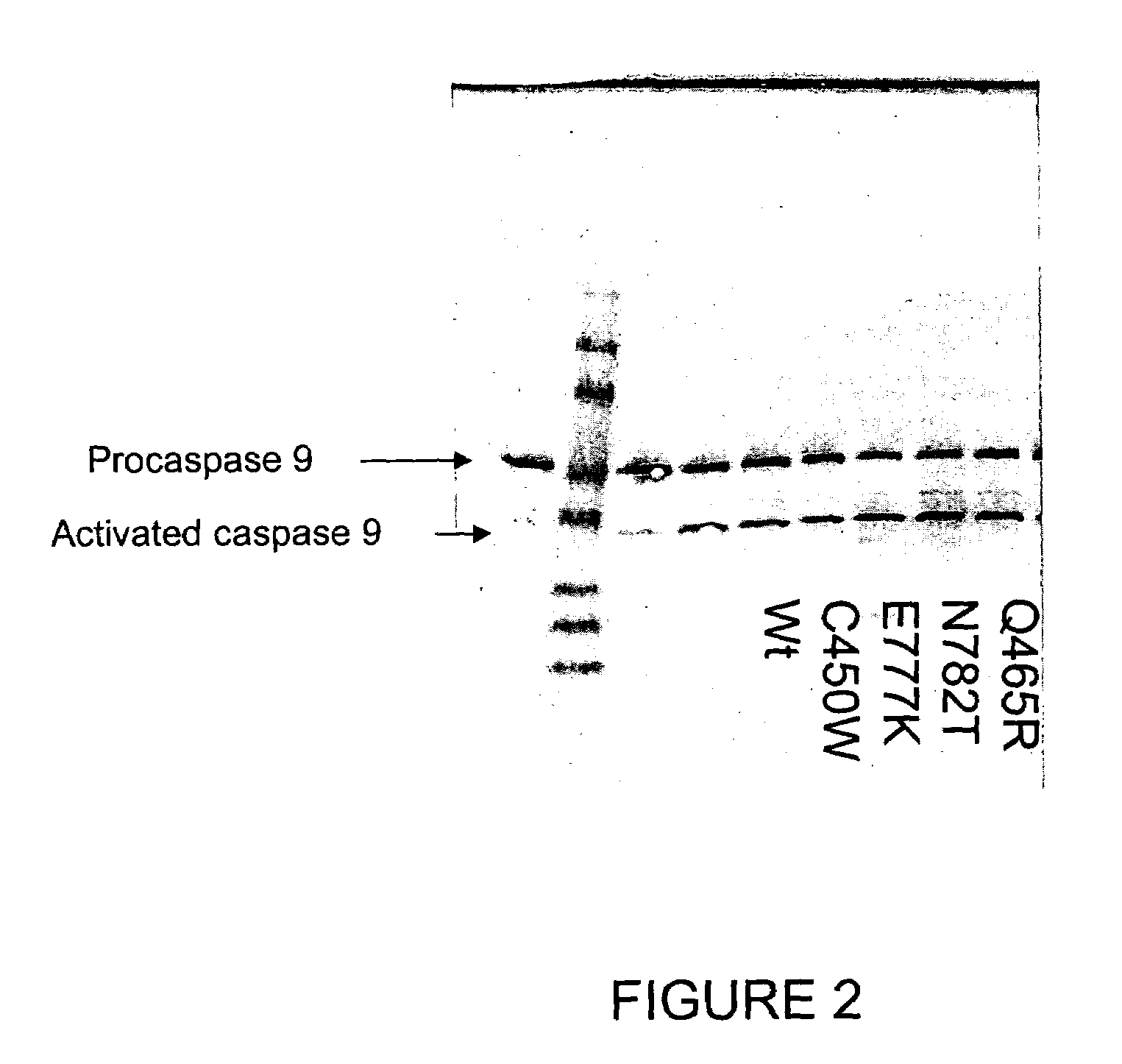
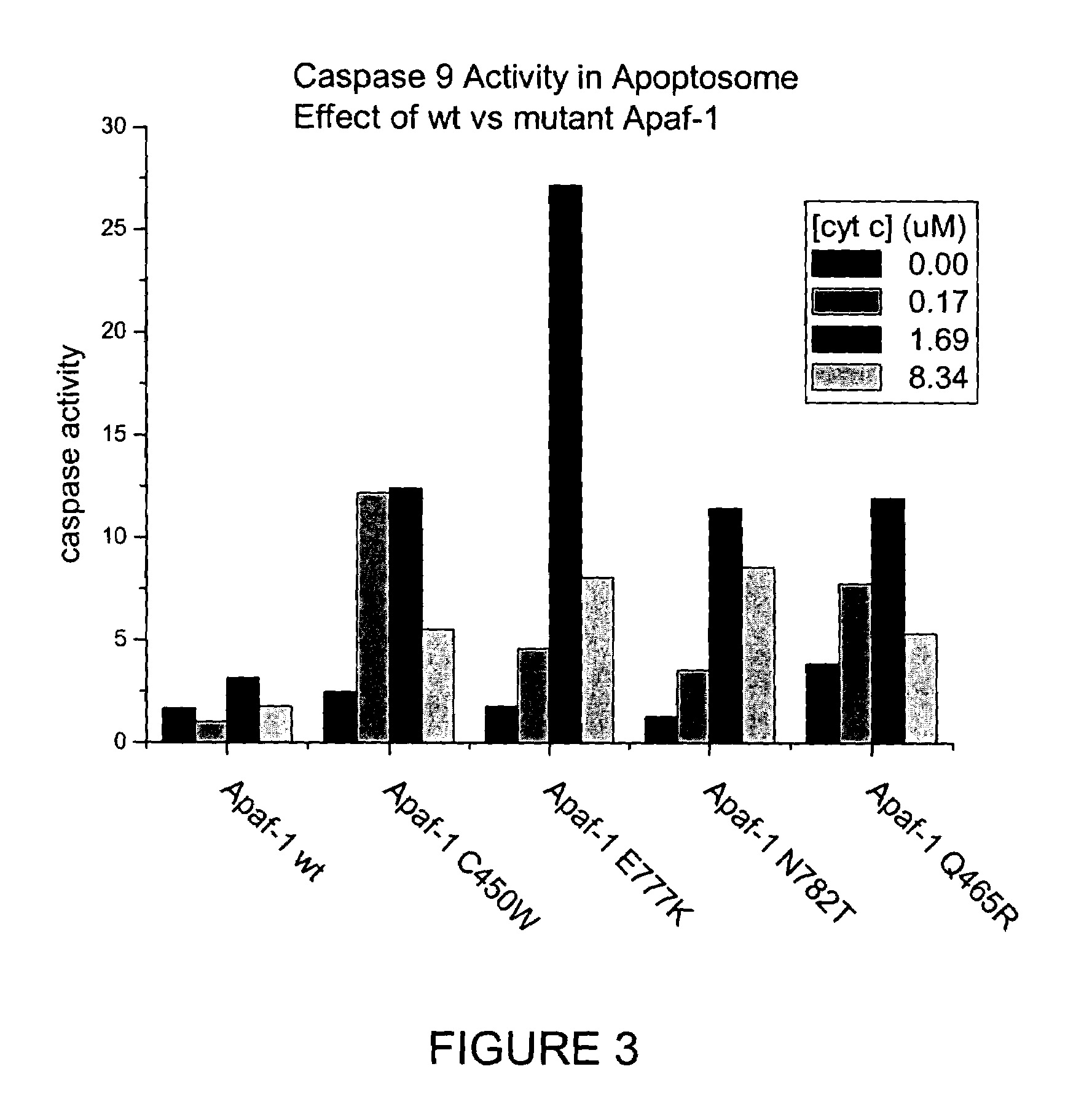
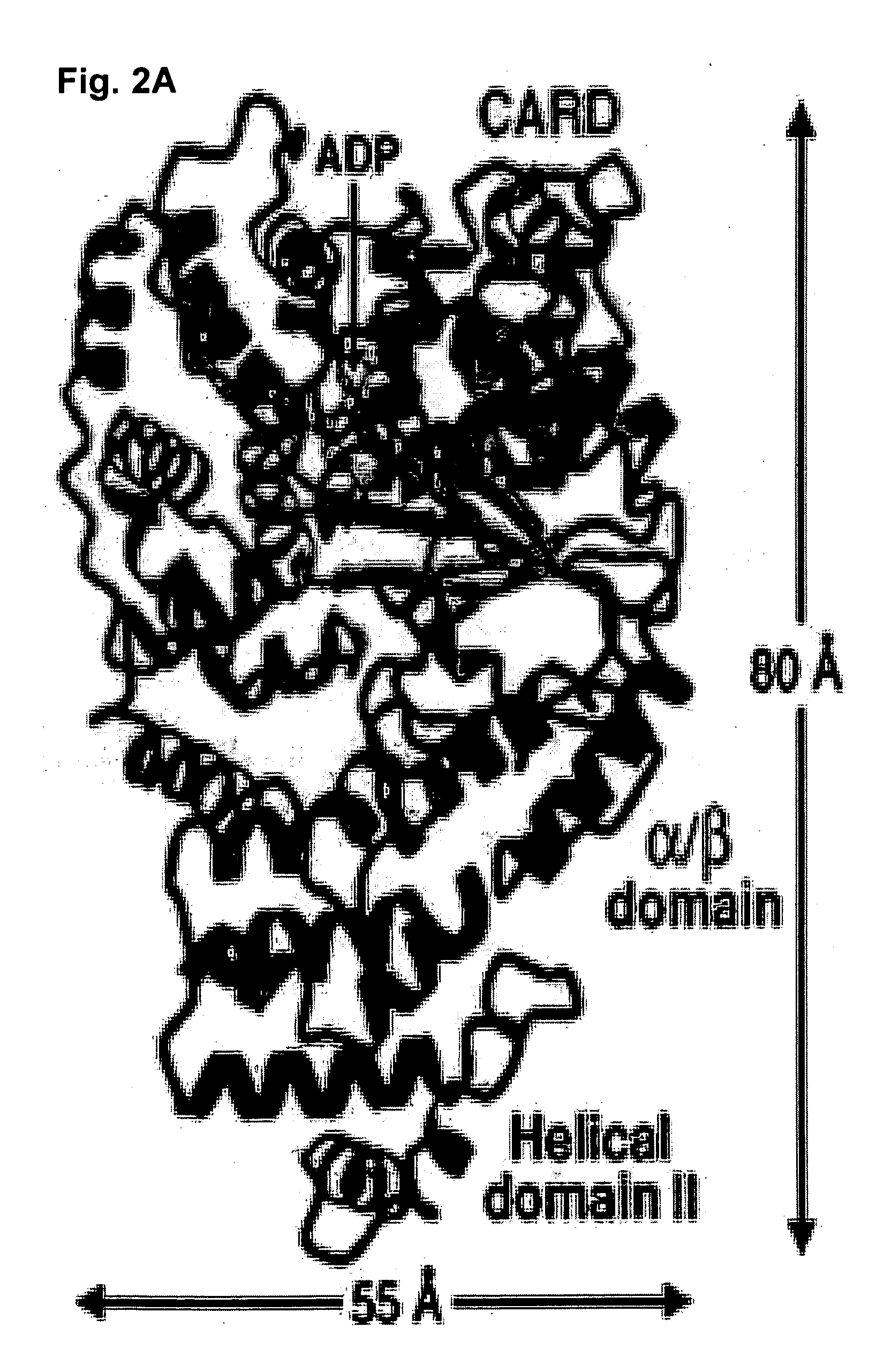
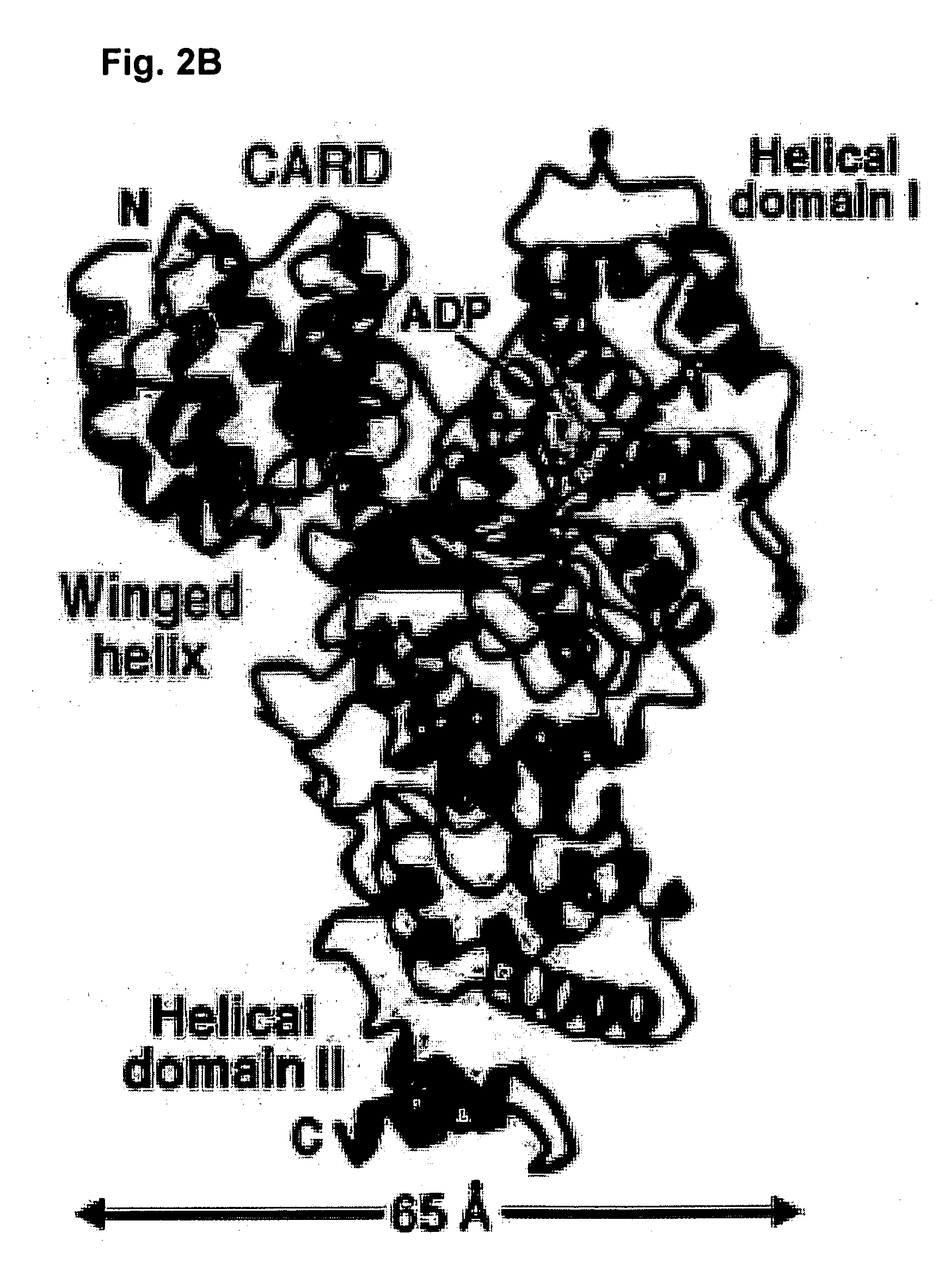
Soluble, functional apoptotic protease-activating factor 1 fragments
The structure of a soluble, functional fragment of human Apaf-1 protein having ADP bound thereto is disclosed. The invention includes such soluble, functional fragments of human Apaf-1 and other metazoan Apaf-1 homologs. Also included in the invention are methods of making such fragments and methods of using them, for example in screening methods to identify adenine nucleotide analogs and other compounds useful for alleviating or preventing disease conditions associated with inappropriate regulation of apoptosis.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Angiogenesis of triple mutant low-oxygen inducible factor 1 alpha induced by auxiliary activating factors and application thereof
InactiveCN102120764AAvoid damageStrong controllabilityFungiBacteriaHistone deacetylaseCoronary heart disease
The invention relates to angiogenesis of a triple mutant low-oxygen inducible factor 1 alpha induced by auxiliary activating factors and application thereof. The invention relates to the triple mutant low-oxygen inducible factor 1 alpha and a vector containing the triple mutant low-oxygen inducible factor 1 alpha for encoding nucleic acid, in particular a recombinant adenovirus vector and a medicinal composition containing the factor and the vector. Triple mutant HIF-1 alpha protein can be bound with the auxiliary activating factors such as response element binding protein (CREB) / adenovirus E1A associated protein P300 (CBP / p300), histone deacetylase (HDAC) and the like to promote expression of HIF-1 alpha downstream target genes such as a vascular endothelial growth factor (VEGF) and the like. The triple mutant protein, the nucleic acid, the vector and the medicinal composition are used for promoting angiogenesis and treating ischemic diseases such as coronary disease, peripheral artery ischemic vascular disease, intermittent claudication and the like.
Owner:吴平生 +2
Depression gene
InactiveUS7052853B1Avoid depressionImprove disease symptomsPeptide preparation methodsDepsipeptidesGermline mutationGenetics human
The present invention relates generally to the field of human genetics. Specifically, the present invention relates to methods and materials used to isolate and detect a human depression predisposing gene, specifically the apoptotic protease activating factor 1 (APAF1) gene, some mutant alleles of which cause susceptibility to depression. More specifically, the invention relates to germline mutations in the APAF1 gene and their use in the diagnosis of predisposition to depression. The invention also relates to the prophylaxis and / or therapy of depression associated with a mutation in the APAF1 gene. The invention further relates to the screening of drugs for depression therapy. Finally, the invention relates to the screening of the APAF1 gene for mutations / alterations, which are useful for diagnosing the predisposition to depression.
Owner:MYRIAD GENETICS
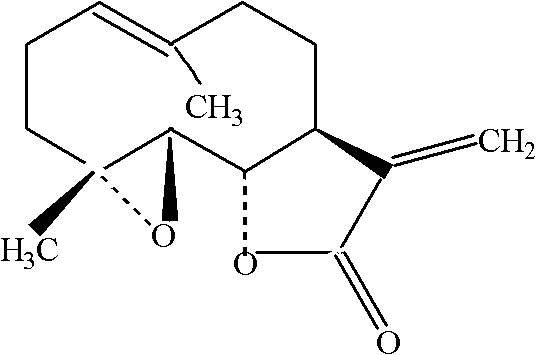
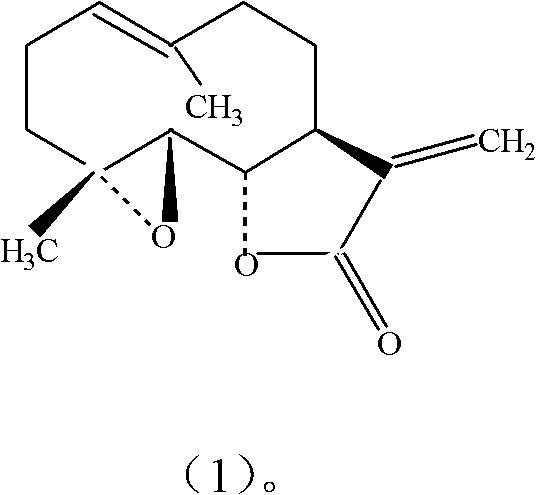
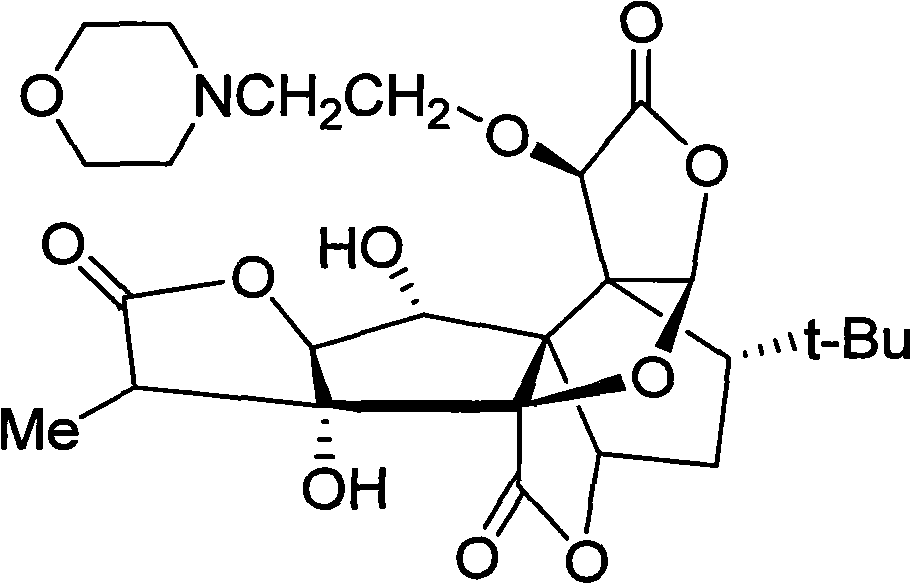
Anti-platelet activating factor compound
InactiveCN101302221AWater Solubility Improvement and EnhancementImprove and enhance bioavailabilityOrganic active ingredientsNervous disorderSolubilityMorpholine
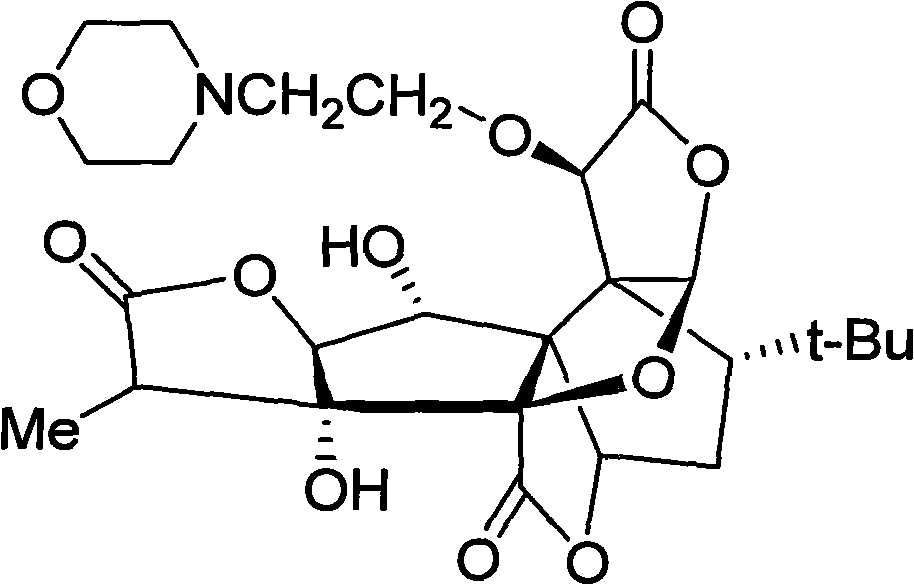
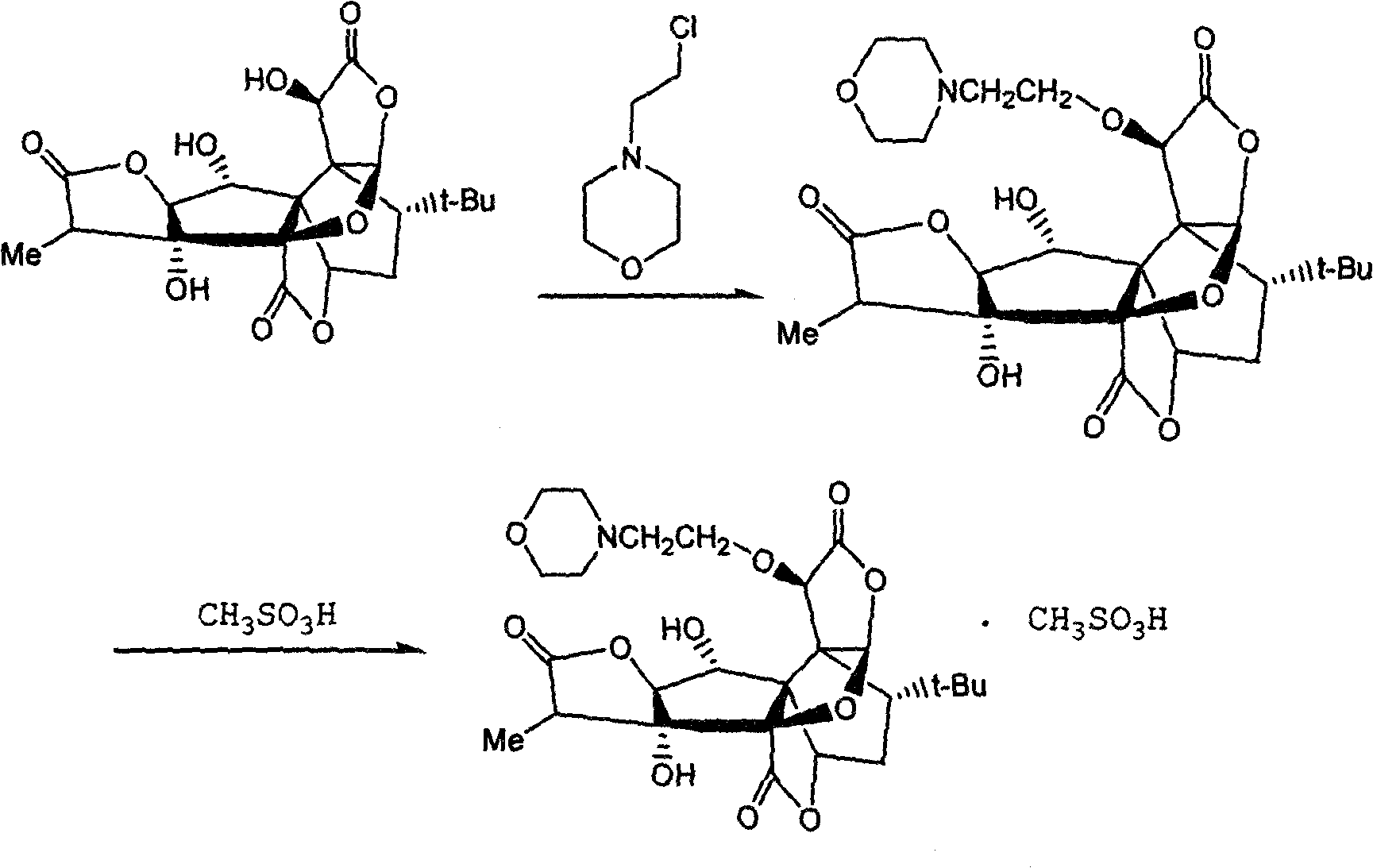
The invention discloses an anti-platelet activation factor compound, wherein the compound is connected with N-ethyl morpholine radical on the NO. 10 oxygen atom of ginkgolide B; the molecular structural formula of 10-O-(N-ethyl morpholine radical) ginkgolide B is shown in the graph. The compound can also be reacted with corresponding organic acid or inorganic acid to generate salt, and can be made into appropriate formulation so as to be applied in clinic. Moreover, the anti-platelet activation factor compound has the advantages that: the compound can increase water solubility and is propitious to bring drug effect into full play; meanwhile, the compound can reduce toxicity and increase safety, etc.
Owner:秦引林
Method for producing clostridium perfringens glycerin anhydrase incitant gene and 1,3-propanediol thereof
InactiveCN101265474ASignificant progressBacteriaMicroorganism based processesEscherichia coliEnzyme Gene
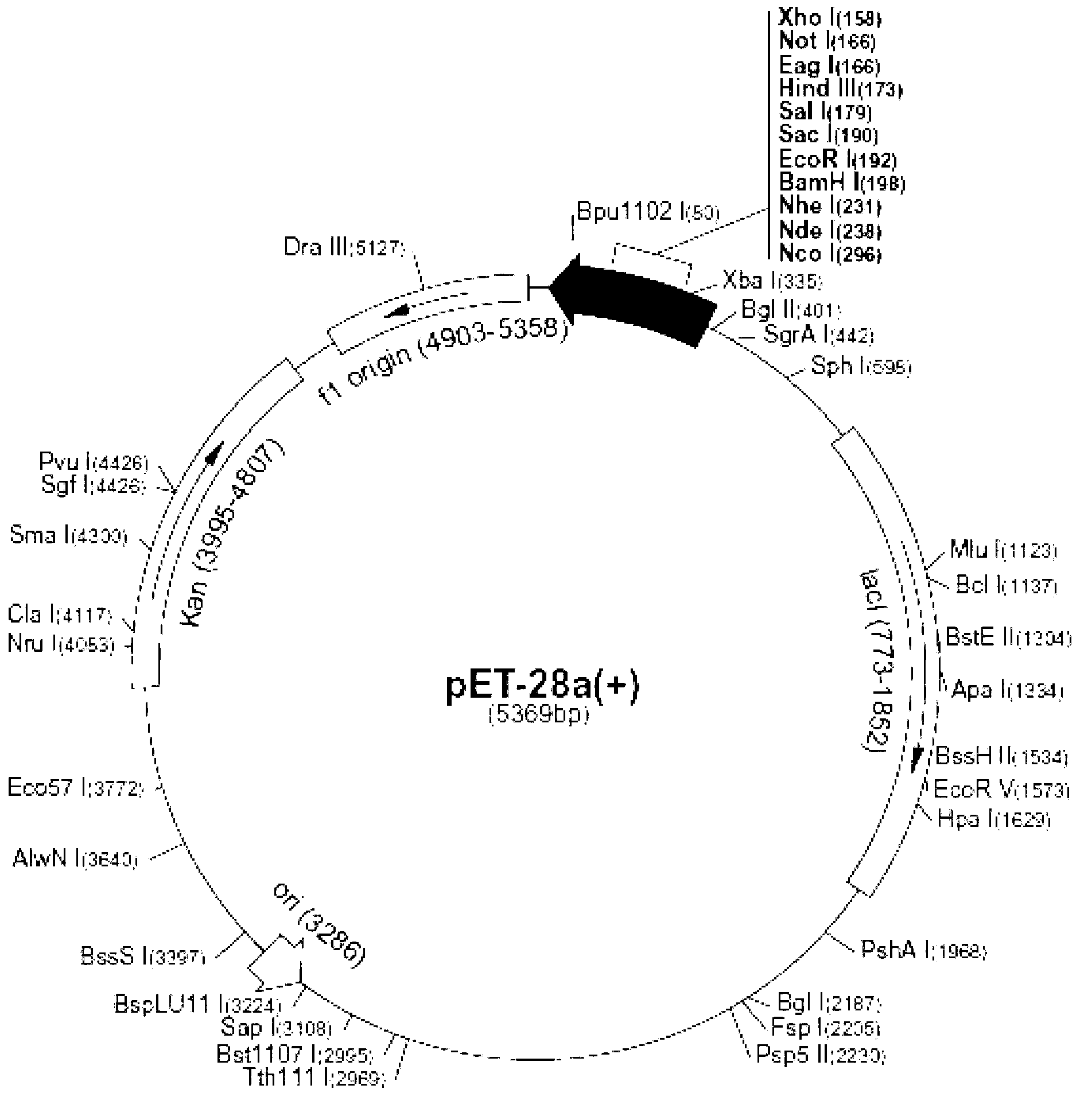
The invention relates to the clone of a novel glycerol dehydratase activating factor gene and the expression thereof in related host. The activating factor gene, cloned from clostridium perfringens, can activate an inactive glycerol dehydratase under normal biochemical reaction conditions and recover the enzyme activity of the glycerol dehydratase, and can also enable the glycerol dehydratase to be difficult to be inactivated when the glycerol dehydratase is transformed to glycerin in the reaction. On the basis of the invention, related enzyme genes, such as the glycerol dehydratase, 1, 3-propanediol oxidoreductase, can be restructured to colon bacillus so as to get novel metabolic engineering bacteria. The metabolic engineering bacteria transform starch, glycerin or glucose to 1, 3-propanediol through fermentation.
Owner:南宁中诺生物工程有限责任公司
Anti-malignant lymphoma fusion protein and preparation method thereof
InactiveCN103172748AGood prospects for tumor treatmentPrevent proliferationPeptide/protein ingredientsPharmaceutical non-active ingredientsMalignant lymphomaMutated protein
The invention provides an anti-malignant lymphoma fusion protein. The anti-malignant lymphoma fusion protein comprises an inhibitor SARI (Suppressor of AP-1 Regulated by Interferon) of a human activator protein-1 and a mutant protein msBAFF of human B cell activating factor BAFF, wherein the msBAFF is a mutant which is formed after amino acids at the 217-224 loci of the human BAFF are replaced by two glycines. The invention further provides a preparation method of the fusion protein. After the recombinant fusion protein of the invention is obtained through a genetic engineering strategy, vitro anti-tumor effect detection is performed and shows that the tumor growth can be effectively inhibited in cells and animals.
Owner:ARMY MEDICAL UNIV
Recombinant anthropogenic hepatocyte growth factor (HGF) activating factor and application thereof
InactiveCN101649320ASignificant inhibition of apoptosisReduce apoptosisPeptide/protein ingredientsDigestive systemDamages tissueTissue repair
The invention relates to the field of biological medicines. A hepatocyte growth factor (HGF) activating factor is plasma protein secreted by hepatocyte, and an activated HGFA is 33Kd polypeptide, is just distributed in the damaged tissues and organs, can activate the monomer HGF secreted by a liver nonparenchyma cell into the HGF at the damaged local part and plays roles of cell protection and tissue repair at the local parts of the damaged tissues and organs. The acute and chronic liver function failure has the main pathological characteristics of large-area hepatocyte apoptosis and necrosisand hepatocyte reproducing function inhibition. The clinical manifestation is dangerous, and the mortality of a patient is as high as 70-90 percent because of lacking an effective curing approach. Theinvention provides a recombinant anthropogenic HGF activating factor which has a nucleotide sequence and a coded amino acid sequence shown as SEQ ID NO:3 and can be used for an application of preparing a medicine for treating acute or subacute severe hepatitis hepatocyte necrosis.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
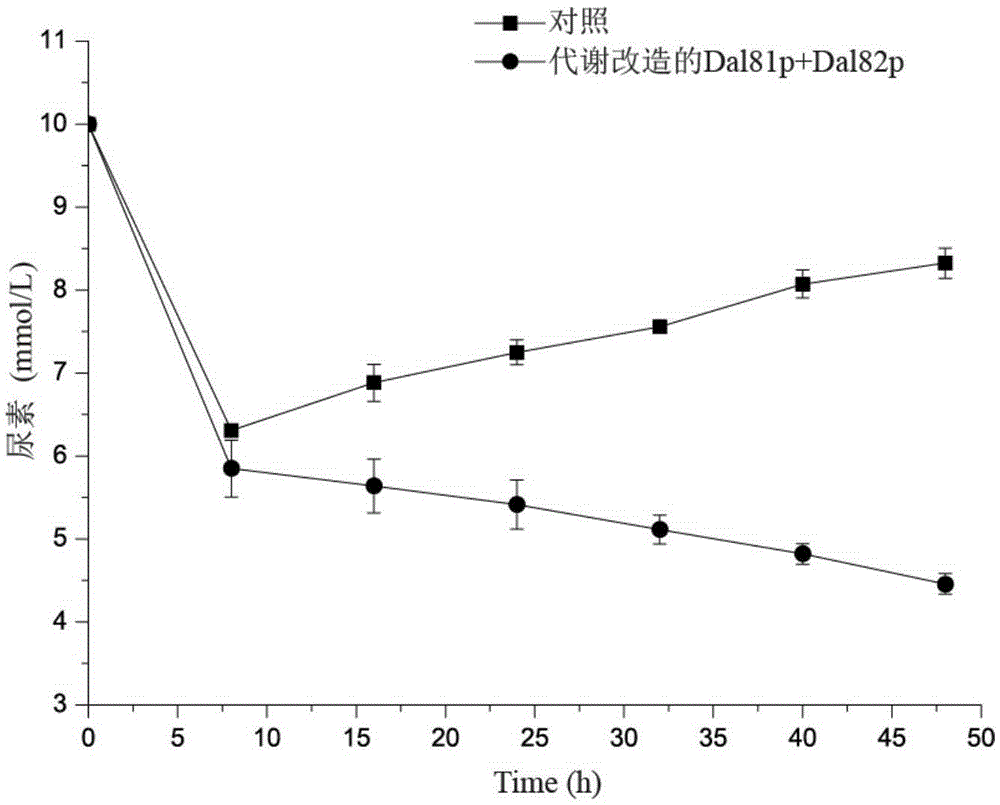
Method for reducing saccharomyces cerevisiae urea accumulation by modifying urea metabolism regulation approach
InactiveCN105274133AReduce accumulationFungiMicroorganism based processesMicrobial GeneticUrea metabolism
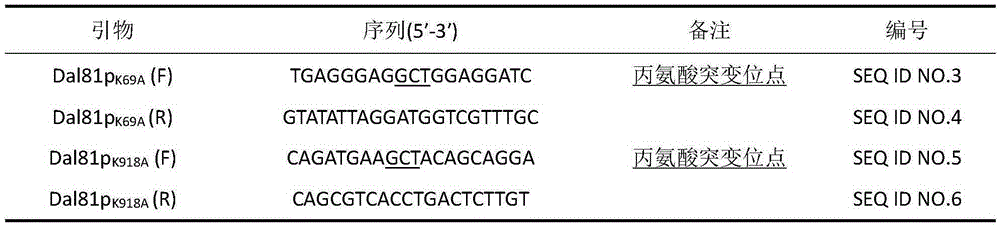
The invention discloses a method for reducing saccharomyces cerevisiae urea accumulation by modifying a urea metabolism regulation approach and belongs to the field of microbial genetics and molecular biology. According to the method, related activating factors of a urea metabolic pathway are modified, and particularly, on the basis of eliminating ubiquitination regulatory sites of Dal81p, Dal81p<K69A>, <K918A> and Dal82p are subjected to overexpression. After the related activating factors of the saccharomyces cerevisiae urea metabolic pathway are subjected to genetic modification according to the method, urea accumulation during saccharomyces cerevisiae fermentation can be reduced fundamentally, and urea accumulation quantity is reduced by 55.7%.
Owner:JIANGNAN UNIV
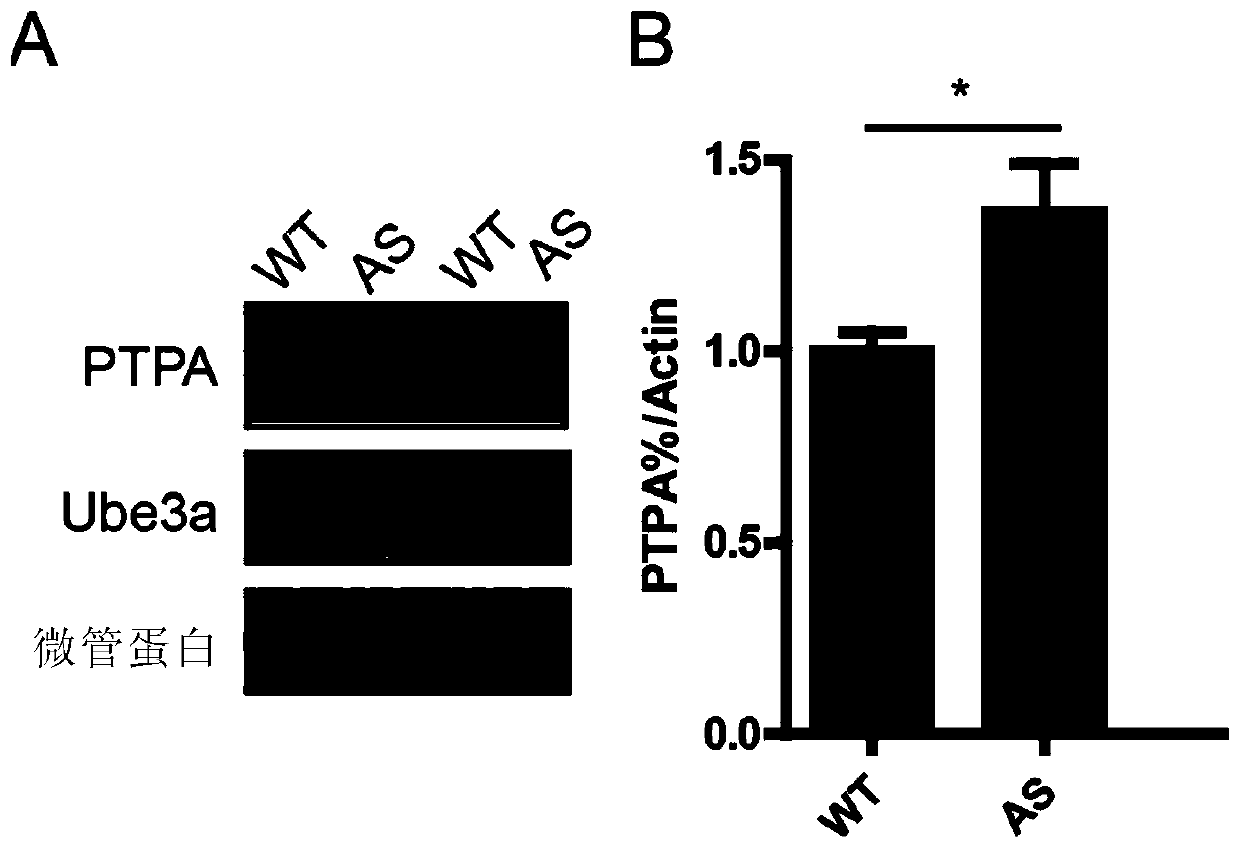
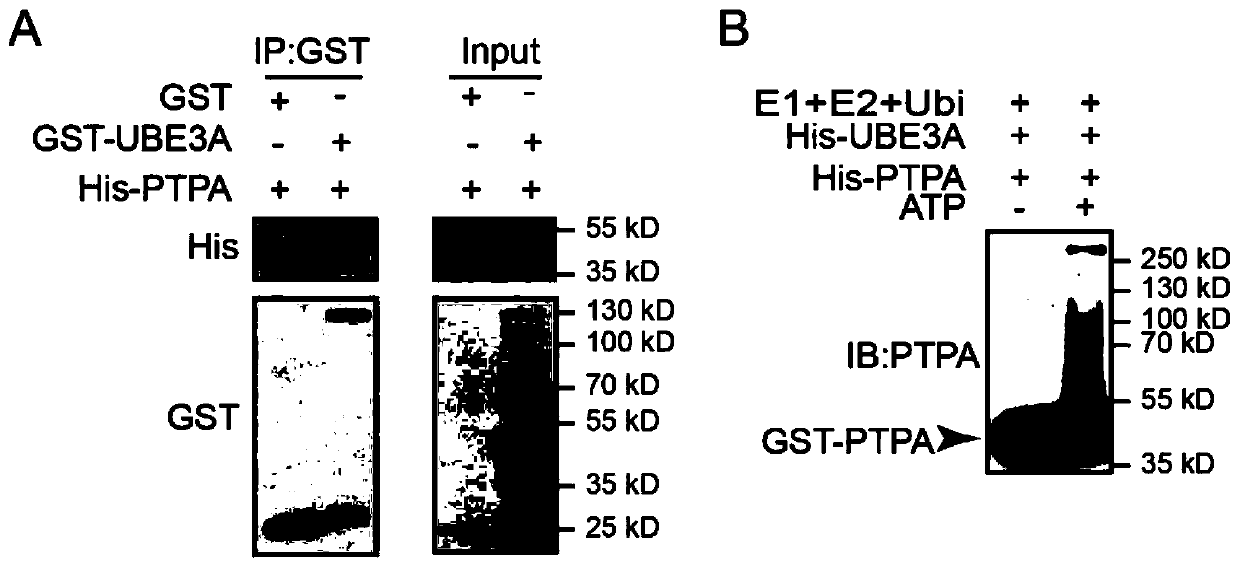
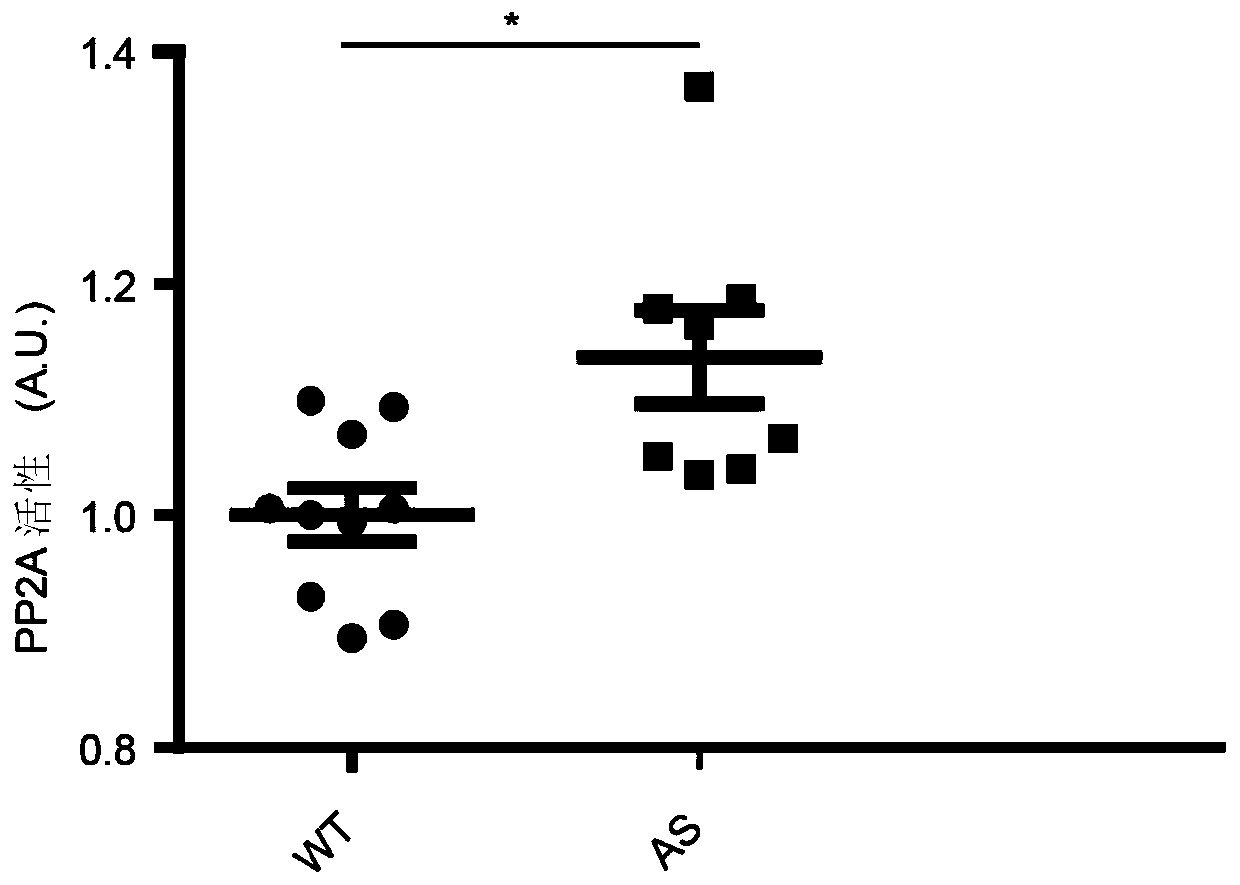
Application of Ube3a-ubiquitinated protein phosphatase 2A (PP2A) activating factor, namely tyrosine phosphatase activating factor (PTPA), for treating Angelman syndrome and autism
The invention relates to application of an Ube3a-ubiquitinated protein phosphatase 2A (PP2A) activating factor, namely a tyrosine phosphatase activating factor (PTPA), for treating Angelman syndrome and autism. The invention discloses new pathogenesis of Angelman syndrome and / or autism or other related diseases; that is to say, lack or over-expression of Ube3a results in abnormal activity of the tyrosine phosphatase activating factor (PTPA) on the protein phosphatase 2A (PP2A). Thus, the factors can be used as targets for developing drugs to alleviate or treat Angelman syndrome and / or autism or other related diseases. The factors can also be used as markers for diagnosing and assessing Angelman syndrome and / or autism or other related diseases.
Owner:CENT FOR EXCELLENCE IN BRAIN SCI & INTELLIGENCE TECH CHINESE ACAD OF SCI
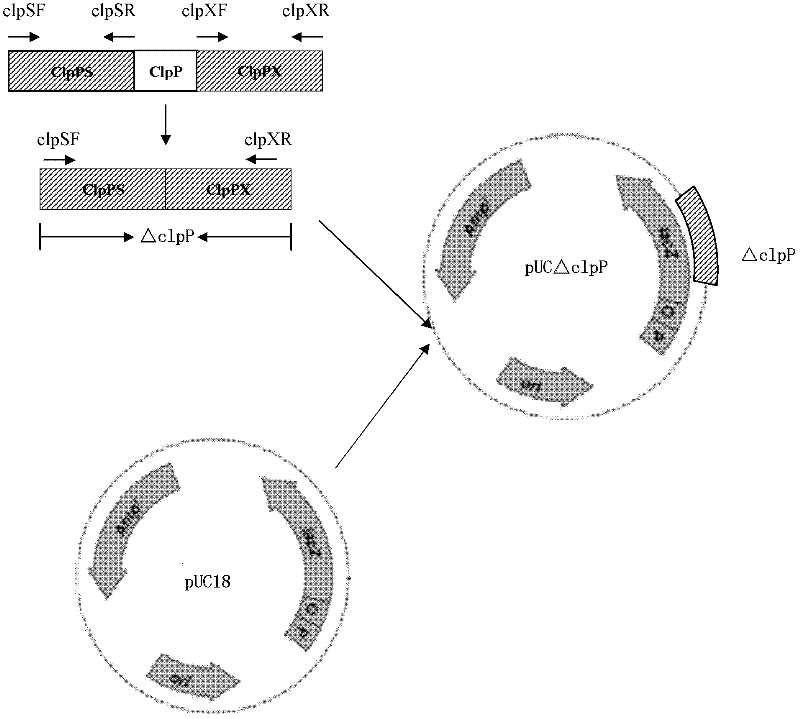
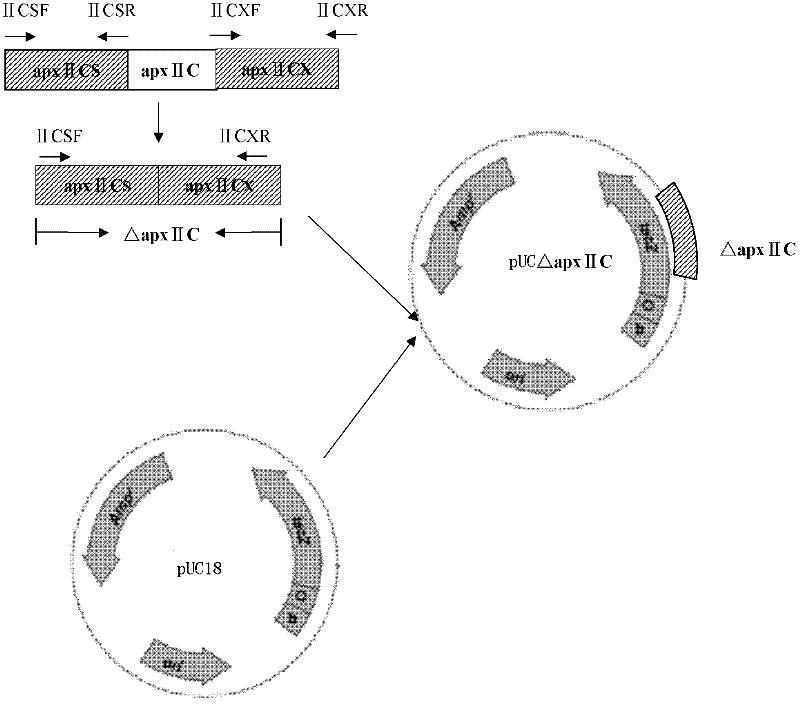
Resistance marker-free porcine actinobacillus pleuropneumoniae double-gene defective strain, construction method and application thereof
InactiveCN102517232AStrong targetingComply with biosafety requirementsAntibacterial agentsBacterial antigen ingredientsBiotechnologyDifferential diagnosis
The invention discloses a resistance marker-free porcine actinobacillus pleuropneumoniae (APP) serum type 7 double-gene defective strain, and belongs to the technical field of bacterial gene engineering. The recombinant strain APPdeltaclpPdeltaapx II C of APP is obtained by inactivating ClpP protease in the APP and a coded gene of a hemolysin activated factor Apx II C by adopting a directional homologous recombination technology, and expression of the ClpP protease and the Apx II C protein is destroyed. The obtained double-gene defective strain has lower toxicity compared with a parent strain, is safe to animals, provides an important basis for transformation of porcine contagious pleuropneumonia (PCP) vaccines and research of matched identification and diagnosis reagents, and has great significance for promoting elimination and purification of the global PCP.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
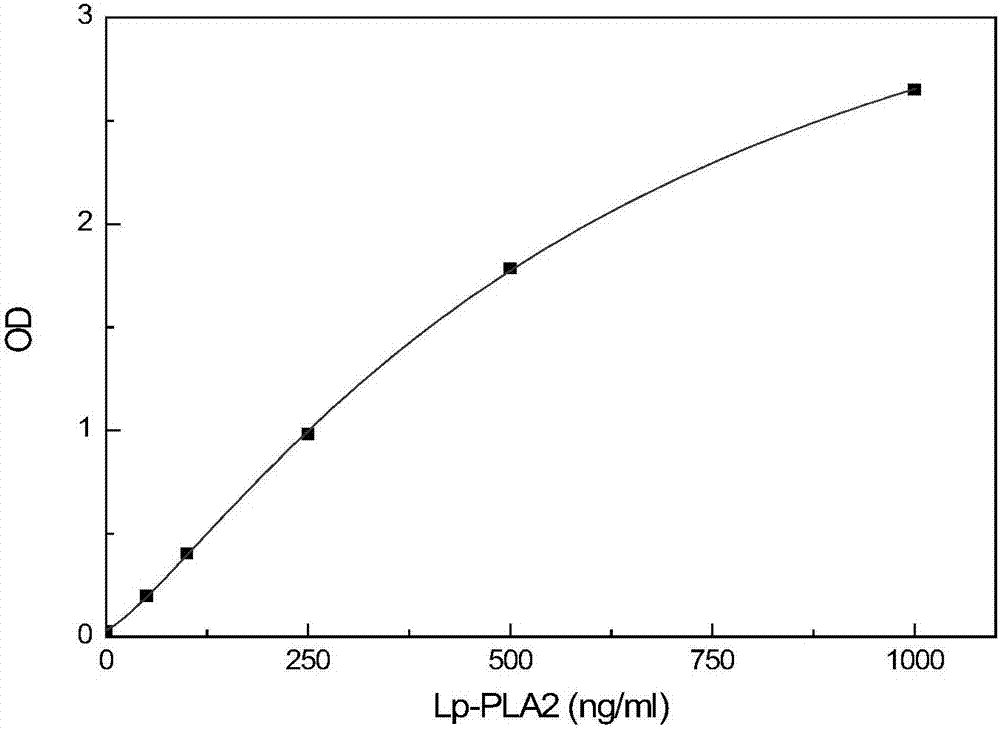
Detection kit for activity and total amount of lipoprotein-associated phospholipase Lp-PLA2 and preparation method thereof
ActiveCN106872687AAccurate judgmentModified Enzyme AssayColor/spectral properties measurementsBiological testingAntigenPhospholipase
The invention discloses a detection kit and a detection method for the activity and the total amount of lipoprotein-associated phospholipase Lp-PLA2. The detection kit is prepared from a solid-phase carrier, a zymolytic substrate, a buffer solution, an enzyme labeled antibody reagent, a cleaning solution, a chromogenic substrate, a stop solution, an enzymatic activity calibration substance and an enzymatic calibration-free substance, wherein the enzyme-linked immunosorbent assay plate is coated with an anti-Lp-PLA2 specific N-terminal non-inhibiting antibody; the zymolytic substrate is a platelet activating factor, a platelet activating factor analogue or phosphatidylcholine modified by the platelet activating factor analogue in an oxidative manner; the enzyme labeled antibody reagent is horseradish peroxidase or alkaline phosphatase or a biotin labeled anti-Lp-PLA2 inhibiting antibody; the enzymatic activity calibration substance comprises multiple concentrations of paranitrophenol standard substances; the enzymatic calibration-free substance comprises multiple reference calibration substances containing Lp-PLA2 antigens. By using the detection kit, the inference of other matter in a sample can be avoided; the in-vivo condition of the Lp-PLA2 can be more accurately reflected; the more accurate judgment basis is provided for clinic.
Owner:海格德生物科技(深圳)有限公司
Soluble, functional apoptotic protease-activating factor 1 fragments
InactiveUS20060106200A1Peptide/protein ingredientsPeptide sourcesProteinase activityScreening method
The structure of a soluble, functional fragment of human Apaf-1 protein having ADP bound thereto is disclosed. The invention includes such soluble, functional fragments of human Apaf-1 and other metazoan Apaf-1 homologs. Also included in the invention are methods of making such fragments and methods of using them, for example in screening methods to identify adenine nucleotide analogs and other compounds useful for alleviating or preventing disease conditions associated with inappropriate regulation of apoptosis.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Kit for quickly detecting expression quantities of related genes of sorafenib chemotherapeutic medicament
InactiveCN102618656AImprove accuracyStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceExtracellular signalTumor vessel
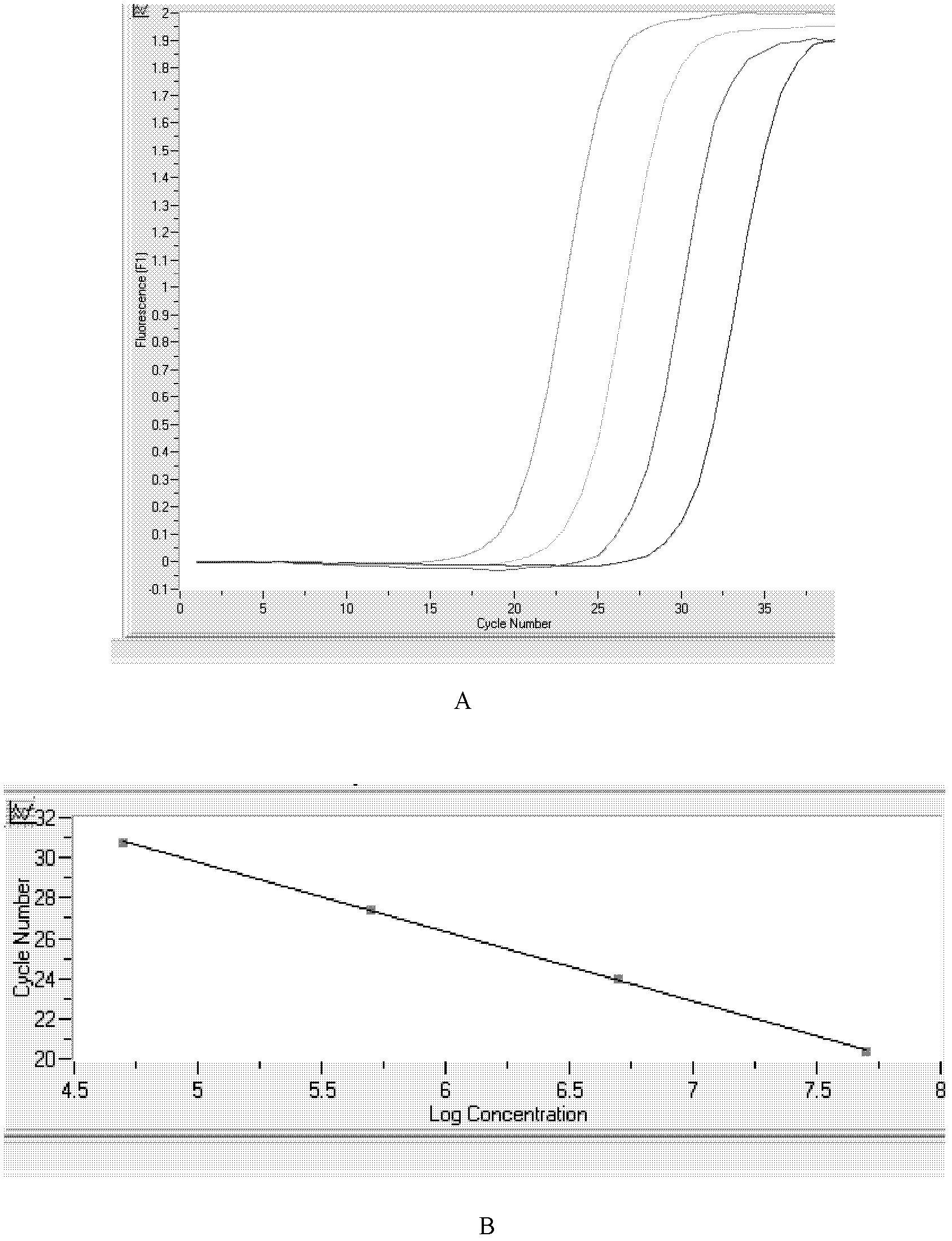
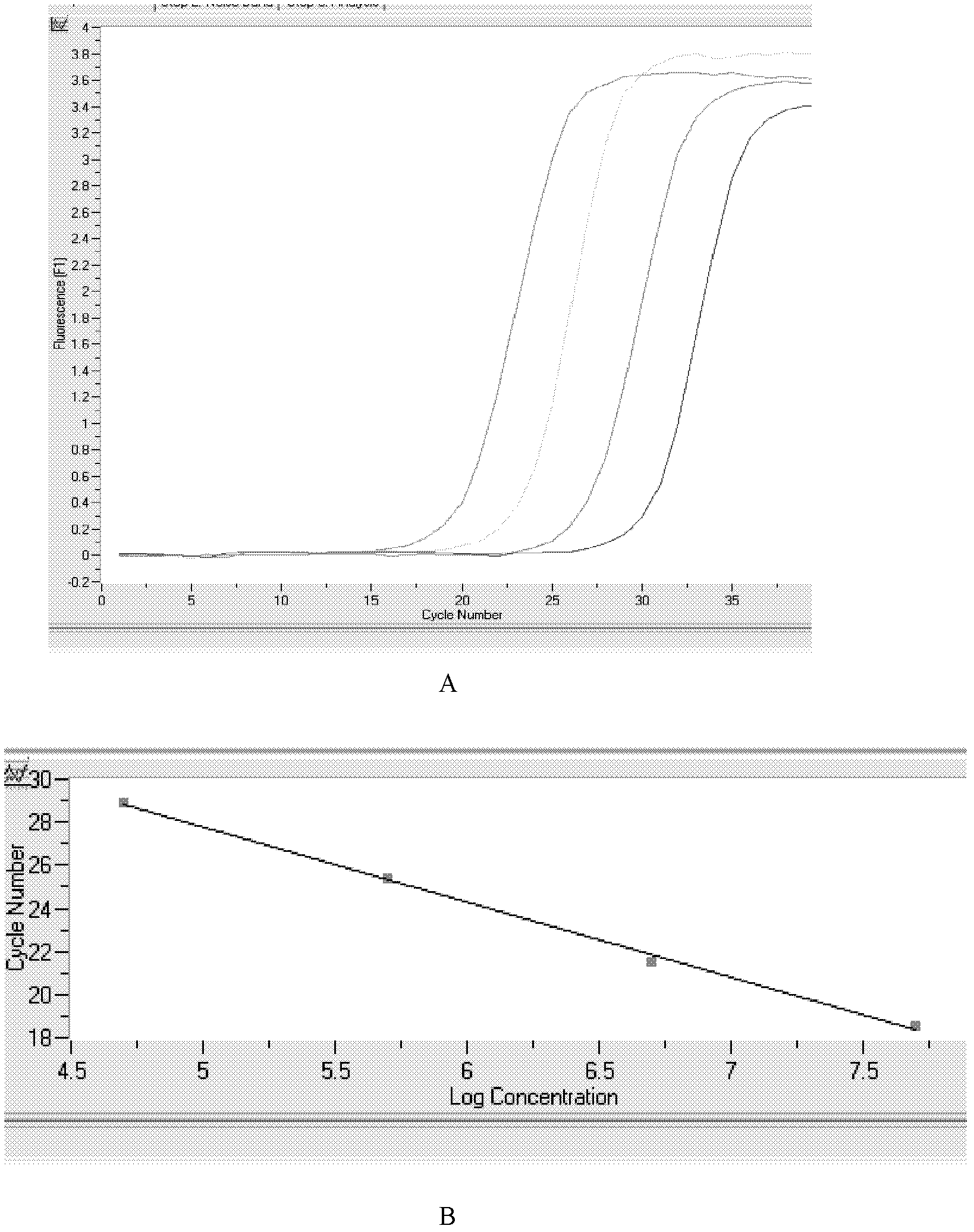
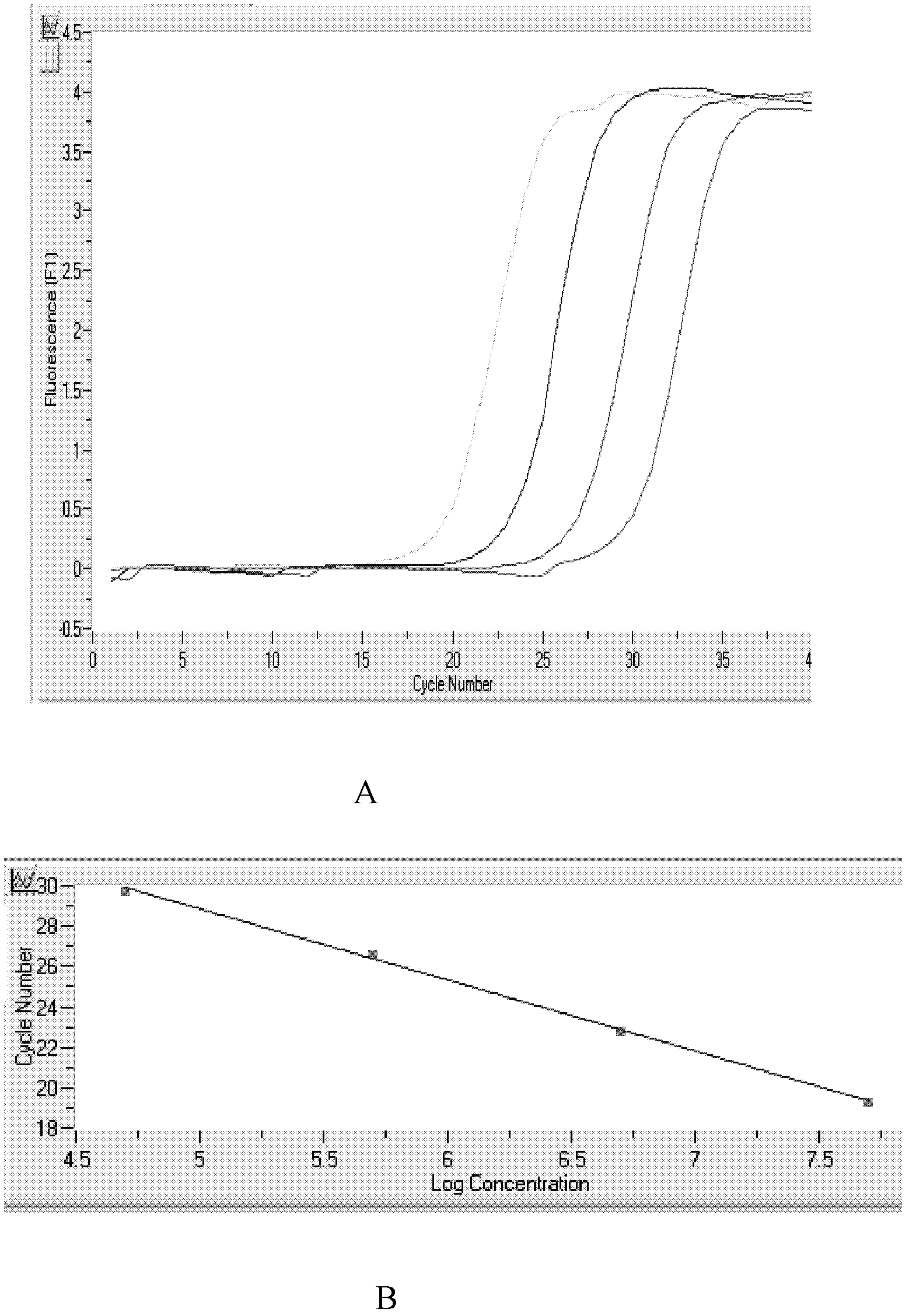
The invention relates to the technical field of biology. High expression of kinase insert domain receptors (KDR) and platelet-derived growth factor receptors alpha (PDGFRA) can be viewed in multiple tumor tissues; and sorafenib directly inhibits tumor growth by inhibiting the activities of the KDR and the PDGFR and inhibiting a recombinant activated factor / methyl ethyl ketone / extracellular signal-regulated kinase (RAF / MEK / ERK) signal transduction pathway, and blocks the formation of new tumor vessels by inhibiting vascular endothelial growth factors (VEGF) and platelet-derived growth factors (PDGF), so that the sorafenib achieves double inhibiting and multi-target blocking anti-hepatic cell carcinoma (HCC) effects. Detection of KDR and PDGFR mRNA levels can assist doctors in forecasting the curative effect of medicaments and the clinical outcome of patients, and has important clinical significance. The invention aims to provide a kit capable of quickly, conveniently, sensitively and specifically detecting expression quantities of related genes KDR and PDGFRA of a sorafenib chemotherapeutic medicament. According to the kit, the KDR and PDGFR mRNA levels are detected by adopting a fluorescent quantitative polymerase chain reaction (PCR) technology with high sensitivity and specificity, so that the sensitivity and the specificity are remarkably improved; and the kit is quick in detection and high in flux, and can finish the detection in 3 to 4 hours.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
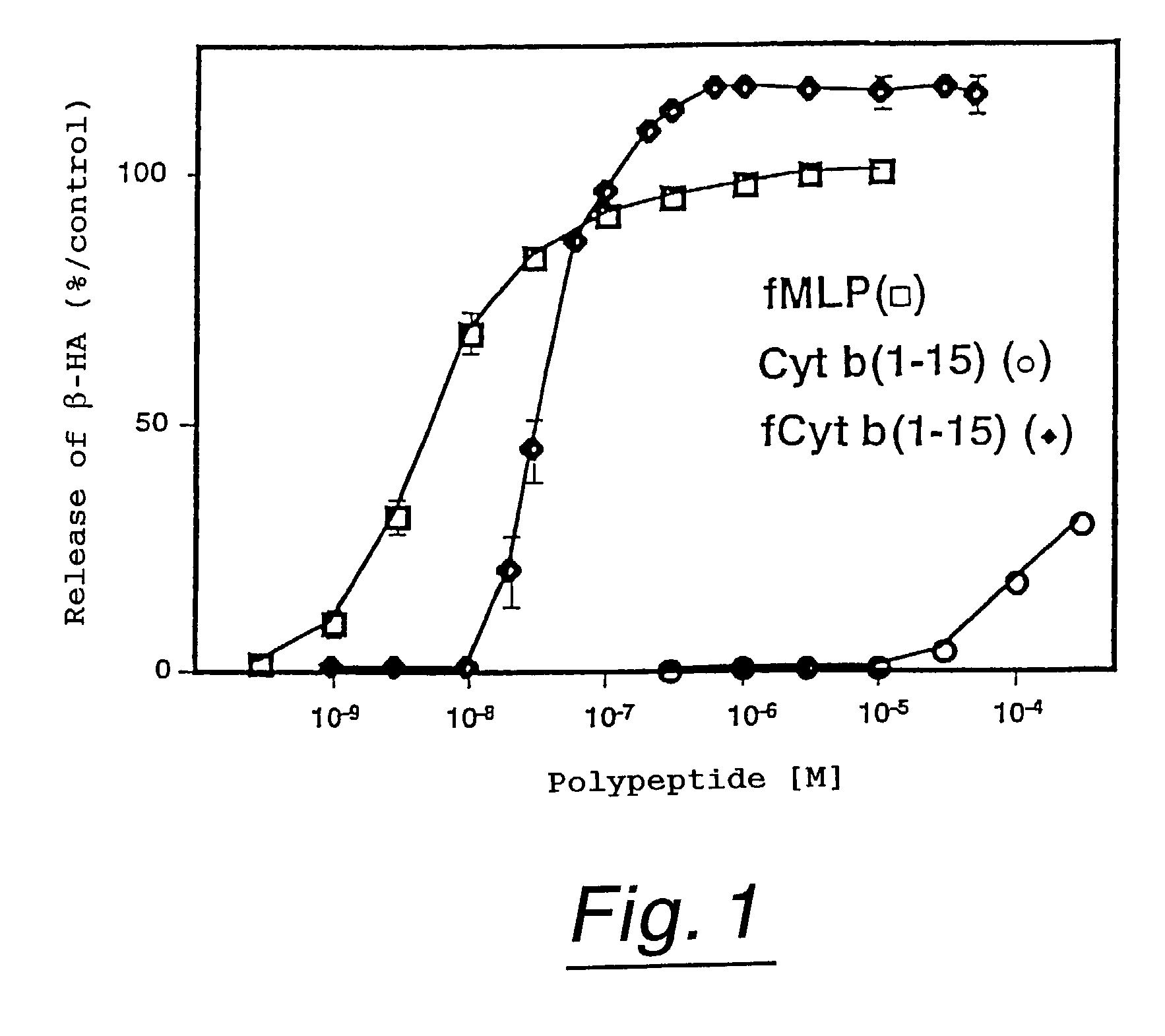
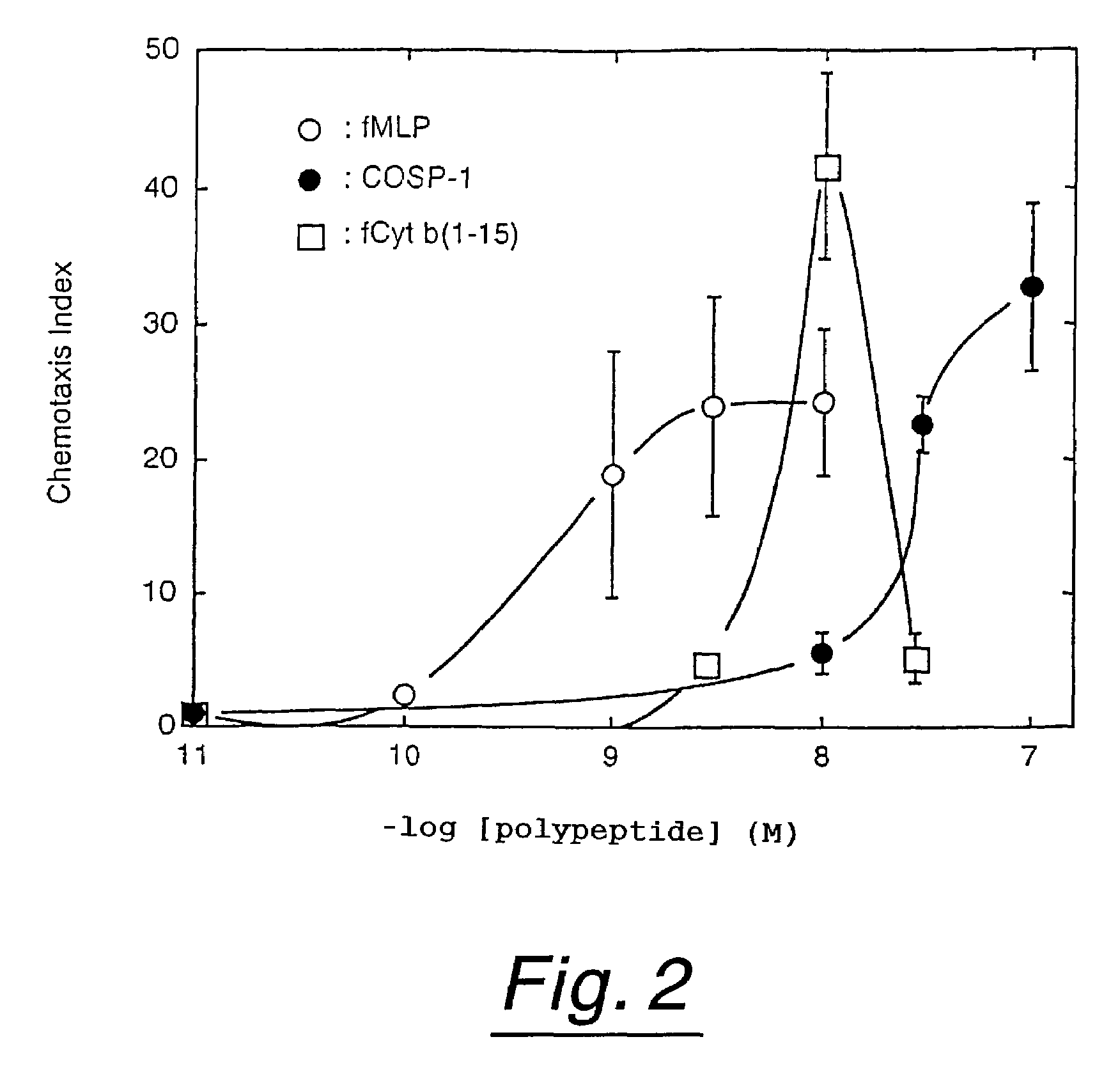
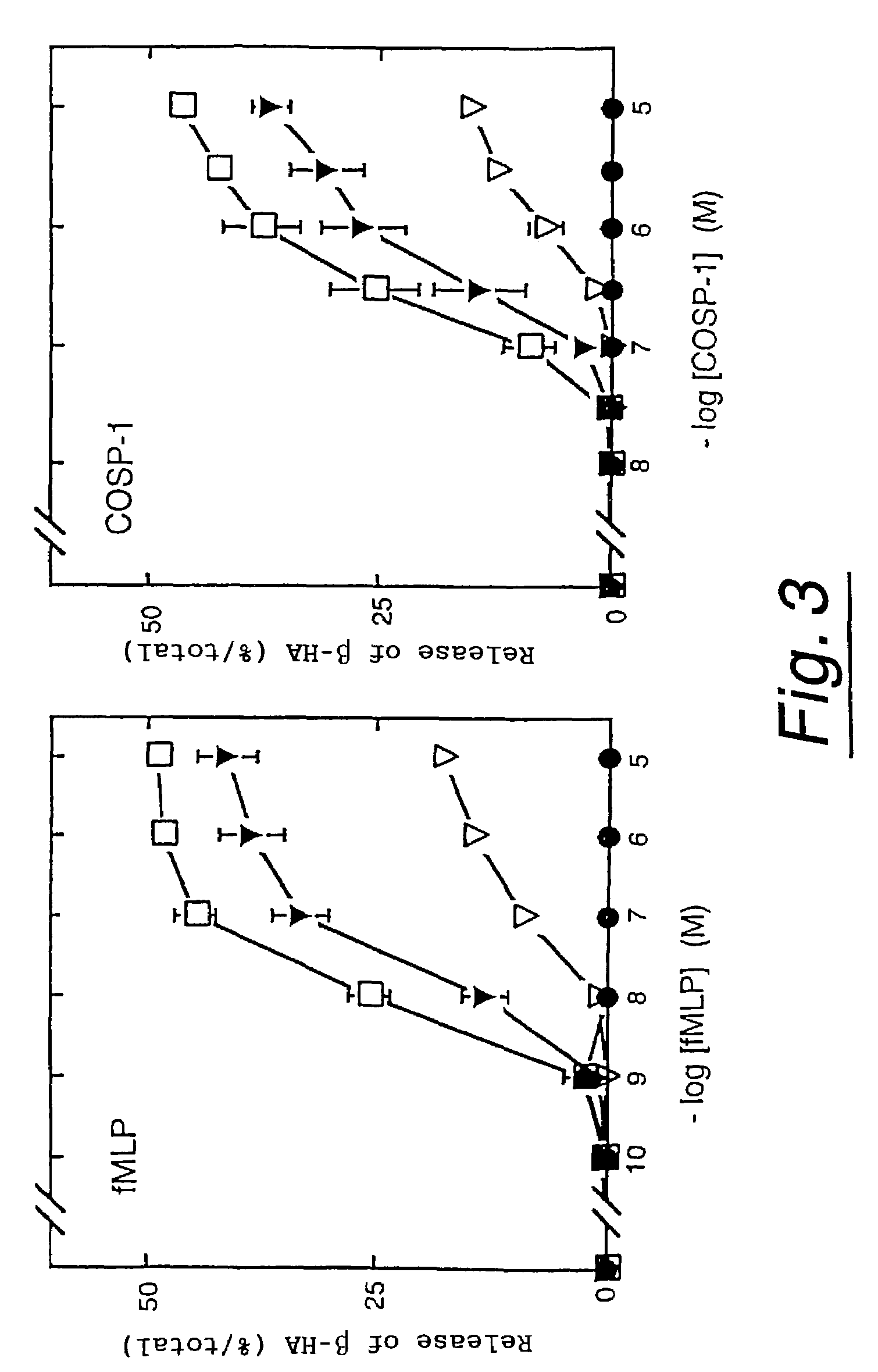
Polypeptides having neutrophil stimulating activity
InactiveUS7285618B1Affect concentrationPeptide/protein ingredientsAntipyreticNormal cellNeutrophil granulocyte
Neutrophil attracting and activating factors that may already exist in normal cells are located.Polypeptides having neutrophil stimulating activity were isolated from an extract originating from a normal porcine heart, whereby the invention was completed. The invention provides (a) a polypeptide consisting of the amino acid sequence of SEQ ID NO:1; (b) a polypeptide consisting of an amino acid sequence derived from the amino acid sequence of SEQ ID NO:3 by deletion, substitution, insertion or addition of one or more amino acids and having neutrophil stimulating activity; or (c) a polypeptide consisting of an amino acid sequence biologically equivalent to the amino acid sequence of said polypeptide (a) or (b).
Owner:JAPAN TOBACCO INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com