Anti-platelet activating factor compound
An activating factor and anti-platelet technology, applied in anti-inflammatory agents, organic chemistry, drug combination, etc., can solve the problems of changing the solubility and efficacy of ginkgolide B, affecting the clinical application effect, and limiting the full efficacy of the drug, etc. Achieve the effect of enhanced bioavailability, low toxicity and enhanced curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
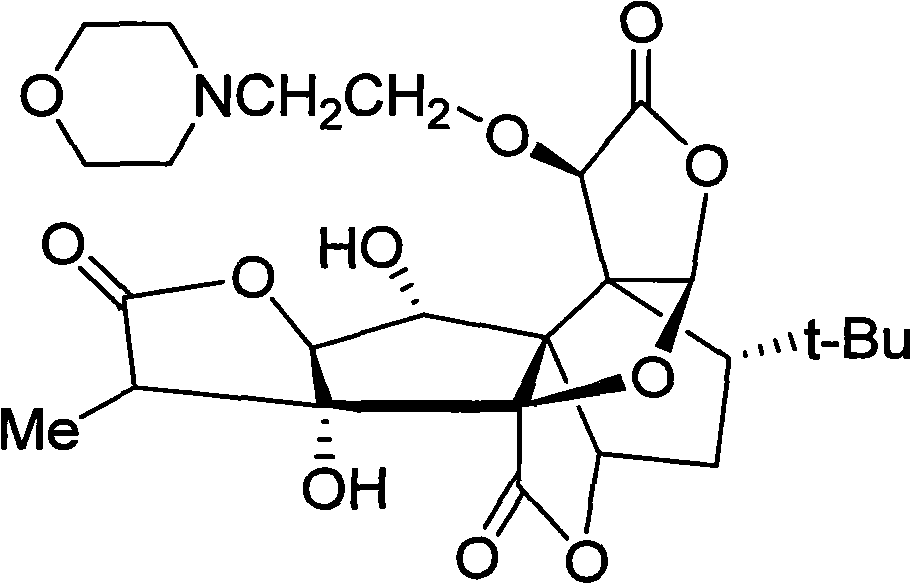
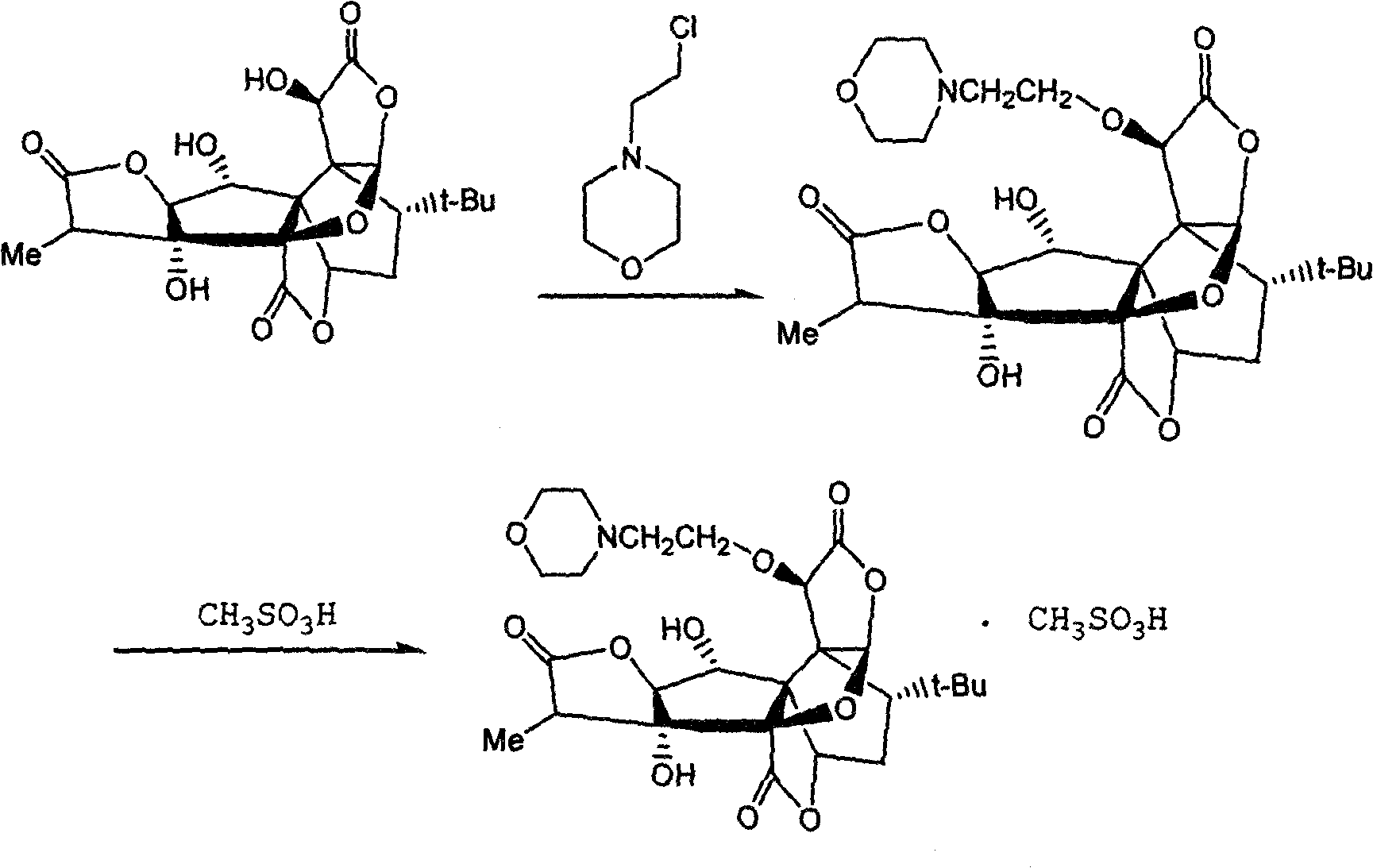
[0014] Example 1 Preparation process of 10-O-(N-ethylmorpholino) ginkgolide B and its mesylate
[0015] 600 mg (1.4 mmol) of ginkgolide B was dissolved in 40 mL of acetonitrile, 370 mg (2.0 mmol) of N-(2-chloroethyl) morpholine hydrochloride, 2.5 g of potassium carbonate, and 50 mg of potassium iodide were added successively, and heated to reflux for 1 hour. The reaction is almost complete. Cool, filter, and concentrate the filtrate under reduced pressure. Add chloroform to the obtained solid and wash it to obtain a crude product. The crude product was chromatographed and eluted with ethyl acetate / petroleum ether (1:1) to obtain a colorless oil. Adding an appropriate amount of anhydrous diethyl ether to the oil resulted in the precipitation of a white solid, which was filtered and dried to obtain 300 mg of a white solid (yield Rate 40%), namely 10-O-(N-ethylmorpholino) ginkgolide B. The obtained solid was dissolved in ethanol, an equimolar amount of ethanol solution of metha...
Embodiment 2
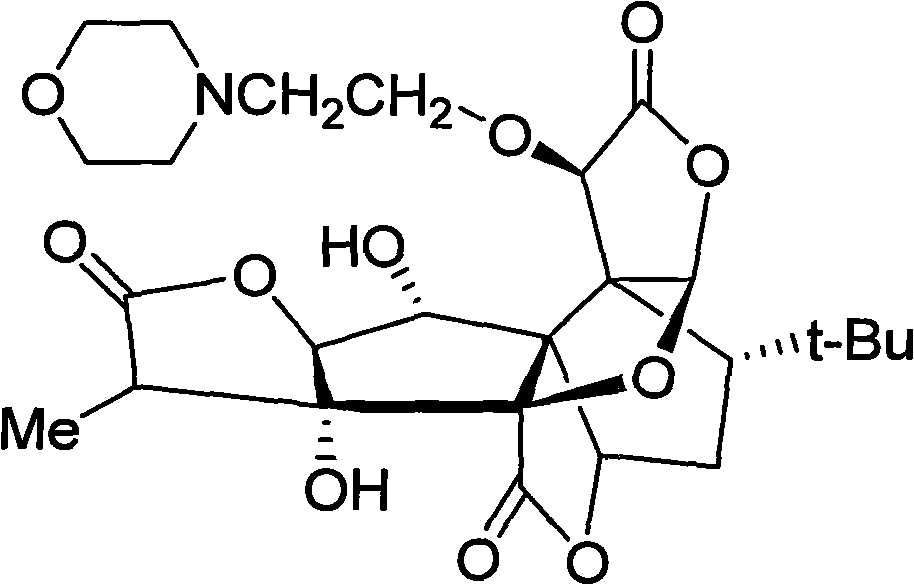
[0018] Example 2 10-O-(N-ethylmorpholino) ginkgolide B acetylsalicylate and its preparation process
[0019] 300 mg of 10-O-(N-ethylmorpholino) ginkgolide B was dissolved in ethanol, and an equimolar amount of acetylsalicylic acid in ethanol was added dropwise, stirred, and spin-dried to obtain 10-O-( N-ethylmorpholino) ginkgolide B acetylsalicylate.
[0020] The synthetic route of 10-O-(N-ethylmorpholino) ginkgolide B acetylsalicylate:
[0021]
Embodiment 3
[0022] Example 3 Comparison of water solubility of compounds before and after modification
[0023] A simple comparison of the water solubility of the compound before and after the structural modification was carried out. Similarly, equimolar amounts of ginkgolide B, 10-O-(N-ethylmorpholinyl) ginkgolide B, 10-O-(N-ethyl Morpholinyl) ginkgolide B hydrochloride and 10-O-(N-ethylmorpholino) ginkgolide B methanesulfonate were measured for their dissolution status, and the results are shown in Table 1.
[0024] Table 1 Comparison results of water solubility of compounds before and after modification
[0025] compound
[0026] It shows that after structural modification, the water solubility of the corresponding salt of ginkgolide B derivatives can change the poor water solubility of original ginkgolide B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com