Anti-malignant lymphoma fusion protein and preparation method thereof
A technology of fusion protein and activation protein, applied in antitumor drugs, peptide/protein components, chemical instruments and methods, etc., can solve problems such as large toxic and side effects, and achieve the effects of high expression, increased productivity, and increased cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Cloning of Human SARI Gene
[0034] 1.1 Isolation of human peripheral blood mononuclear cells
[0035] (1) Take 5ml of anticoagulated blood and mix it with PBS buffer 1:1.
[0036] (2) Take 10 ml of lymphocyte separation fluid (China TBD Biotechnology Development Center).
[0037] (3) Use a dropper to carefully add the mixture obtained in (1) to the liquid surface of the separation liquid obtained in (2), and let stand at room temperature for 5 minutes.
[0038] (4) Centrifuge horizontally at 2000 rpm for 20 minutes to collect the buffy coat.
[0039] (5) The cells were washed twice with PBS buffer, centrifuged at 2000 rpm for 10 minutes, and the supernatant was discarded. The resulting pellet contained human peripheral blood mononuclear cells.
[0040] 1.2 Extraction of total cellular RNA
[0041] Trizol was used to extract total cellular RNA, and the operation steps were carried out according to the instructions of Trizol reagent.
[0042] (1) In the ...
Embodiment 2
[0061] Example 2 Induced expression and identification of target protein
[0062] 1. Expression of target protein
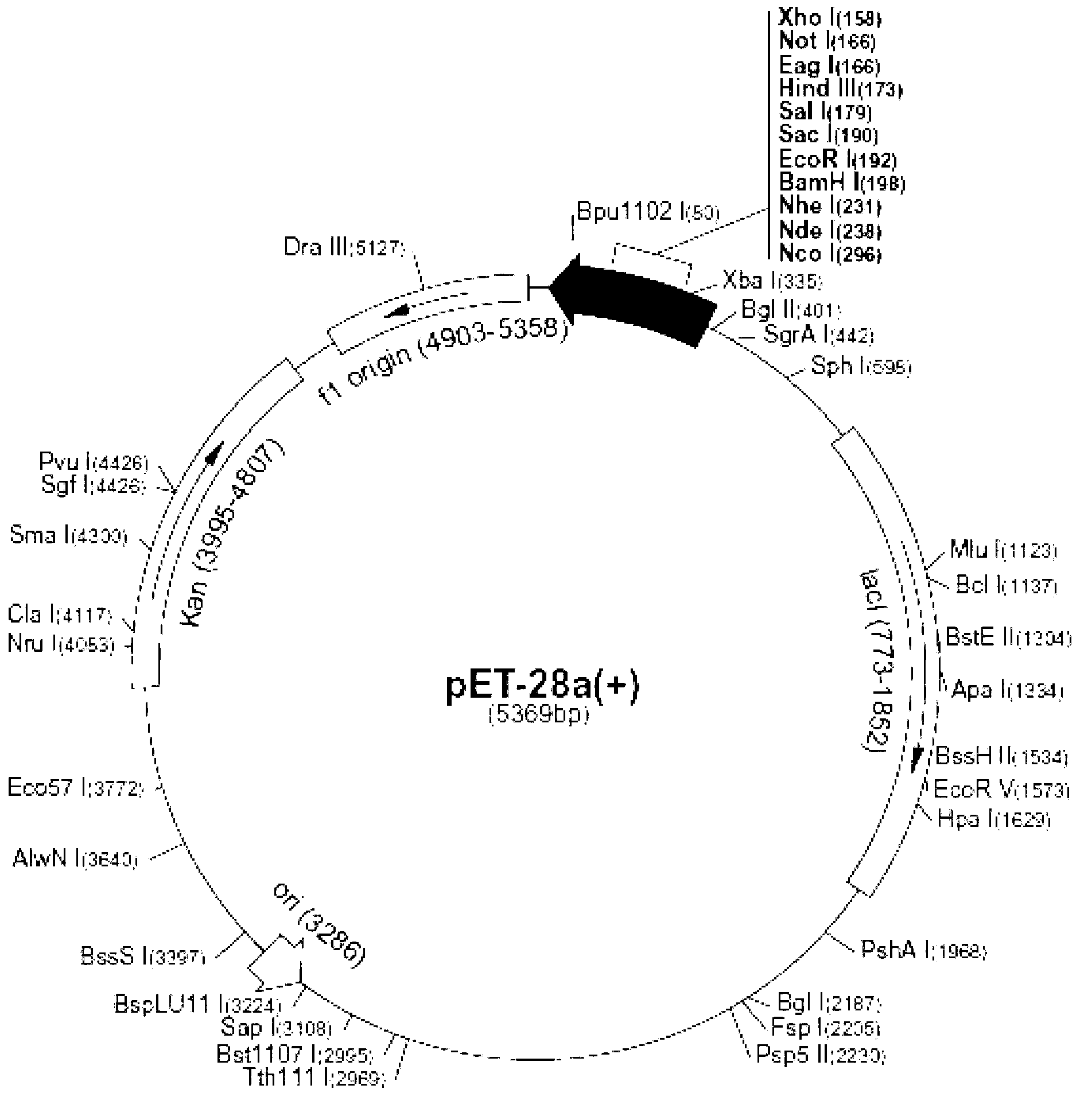
[0063] (1) Pick a single colony containing the recombinant prokaryotic expression plasmid pET-SGRB and the control plasmid pET28a, and inoculate them in 3ml LB liquid medium (containing 50μg / ml Amp), respectively, and culture overnight at 37°C and 220rppm with shaking.
[0064] (2) Inoculate 1% of the bacterial solution cultured by shaking overnight into 5ml LB liquid medium (containing 50μg / ml Amp), shake and culture at 37°C and 250r / min for 3 hours until mid-logarithmic phase [OD(600) ≈0.6].
[0065] (3) Add IPTG to a final concentration of 1 mmol / L, induce expression at 37°C and 220r / min for 4 hours.
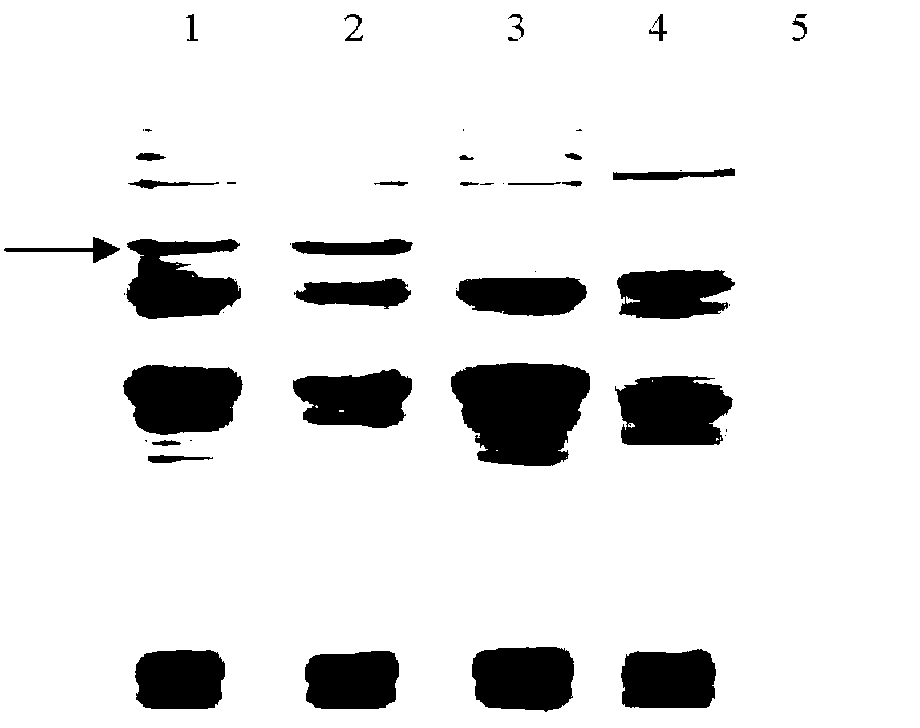
[0066] (4) SDS-PAGE to detect the expression of the target protein.
[0067] 2. Denaturing polyacrylamide gel electrophoresis (SDS-PAGE)
[0068] (1) Take 1ml of the bacterial solution after induction of expression, centrifuge at 10000×g for 1min, and disc...
Embodiment 3
[0097] Example 3 Isolation and purification of target protein
[0098] 1. Mass expression of target protein
[0099] (1) Escherichia coli containing the recombinant prokaryotic expression plasmid pET-SGRB was inoculated in 5 ml of LB culture solution (containing 50 μg / ml Amp), shaken overnight at 37° C. and 220 rpm.
[0100] (2) Inoculate 500ml of LB culture solution containing 100μg / ml ampicillin at 1% of the bacterial solution shaken overnight, shake vigorously at 37°C for 3 hours, until mid-logarithmic phase [D(600)≈0.6].
[0101] (3) Add IPTG to a final concentration of 1 mmol / L.
[0102] (4) Continue shaking at 20°C for 24 hours.
[0103] (5) Bacteria were collected by centrifugation at 4000g for 15 minutes at 4°C.
[0104] 2. Identification of recombinant protein expression
[0105] (1) Add 3ml TE buffer (pH8.0) per gram of bacteria.
[0106] (2) Sonicate the bacteria in an ice bath until the viscous bacterial solution becomes clear.
[0107] (3) Centrifuge at 4°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com