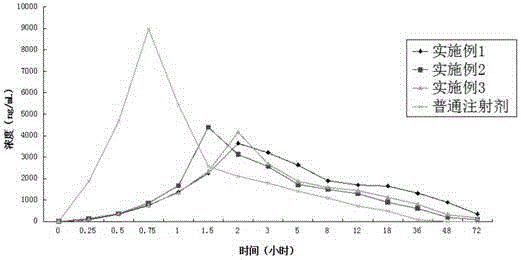
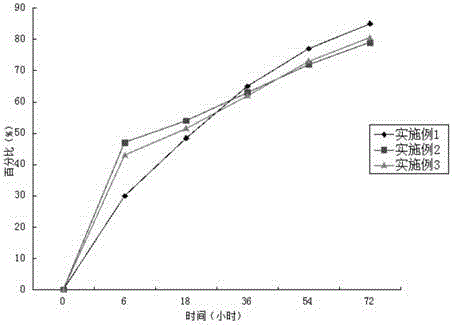
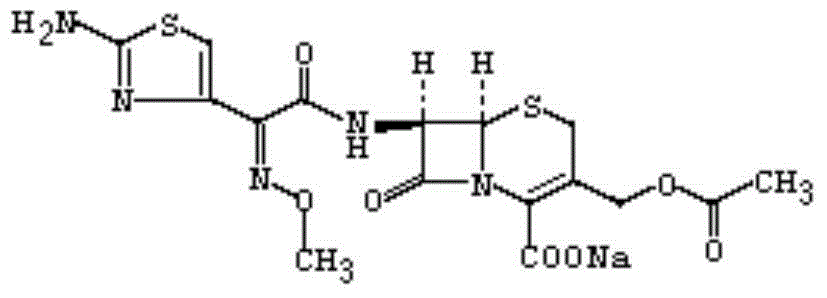
Long-acting cefotaxime sodium injection and preparation method thereof
A technology of cefotaxime sodium and injection, which is applied in the field of long-acting cefotaxime sodium injection, can solve problems such as no reports, and achieve the effects of prolonging drug effect, reducing occurrence probability, and reducing the number of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Prepare 30 mg / mL N-succinyl chitosan solution, carboxymethyl chitosan solution, and oxidized chondroitin sulfate solution respectively, and add 1 g of cefotaxime sodium to 50 mL of In the N-succinyl chitosan solution, keep stirring, slowly add 40mL carboxymethyl chitosan solution after 30min, keep stirring, add 60mL oxidized chondroitin sulfate solution and 20mL glutaraldehyde with a concentration of 50% at a uniform speed after 25min The solution was added within 2 minutes at a constant speed, mixed for 1 hour at a stirring speed of 5000 RPM, injected into a circular mold with a bottom diameter of 10 mm to form a drug-loaded hydrogel, and vacuum-dried at room temperature to a constant weight to obtain a dry load. Potion gel.
Embodiment 2
[0037] Prepare 30 mg / mL N-succinyl chitosan solution, carboxymethyl chitosan solution, and oxidized chondroitin sulfate solution respectively, and add 1 g of cefotaxime sodium to 70 mL of In the N-succinyl chitosan solution, keep stirring, slowly add 20mL carboxymethyl chitosan solution after 30min, keep stirring, add 60mL oxidized chondroitin sulfate solution and 20mL glutaraldehyde with a concentration of 50% at a uniform speed after 25min The solution was added within 2 minutes at a constant speed, mixed for 1 hour at a stirring speed of 5000 RPM, injected into a circular mold with a bottom diameter of 10 mm to form a drug-loaded hydrogel, and vacuum-dried at room temperature to a constant weight to obtain a dry load. Potion gel.
Embodiment 3
[0039] Prepare 30 mg / mL N-succinyl chitosan solution, carboxymethyl chitosan solution, and oxidized chondroitin sulfate solution respectively, and add 1 g of cefotaxime sodium to 30 mL of In the N-succinyl chitosan solution, keep stirring, slowly add 60mL carboxymethyl chitosan solution after 30min, keep stirring, add 60mL oxidized chondroitin sulfate solution and 20mL glutaraldehyde with a concentration of 50% at a uniform speed after 25min The solution was added within 2 minutes at a constant speed, mixed for 1 hour at a stirring speed of 5000 RPM, injected into a circular mold with a bottom diameter of 10 mm to form a drug-loaded hydrogel, and vacuum-dried at room temperature to a constant weight to obtain a dry load. Potion gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com