Preparation method of 3,4-polybeta-myrcene
A kind of technology of myrcene and isoprene, applied in the field of preparation of 3,4-poly-β-myrcene
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0068] Preparation of catalyst combination:
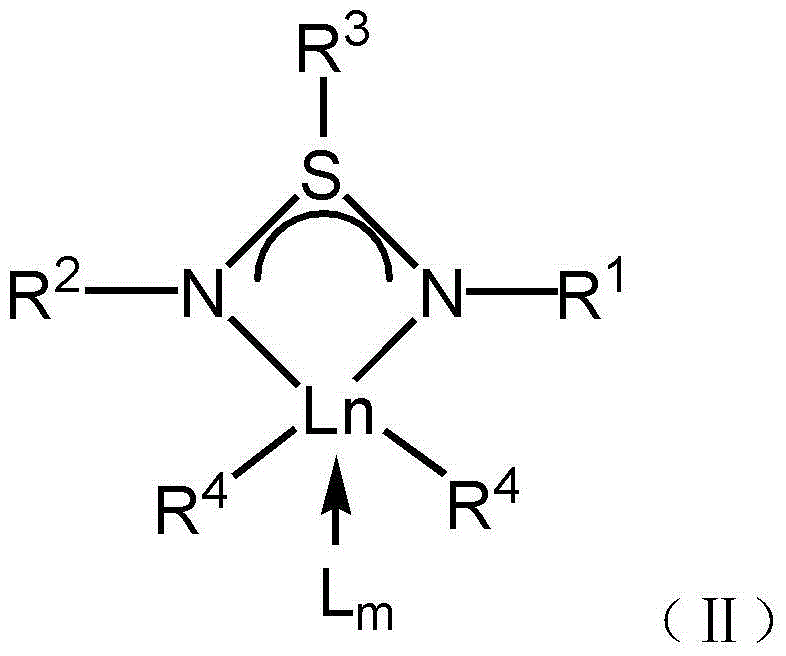
[0069] Preparation of Catalyst Combination 1: At 25°C, add 10 μmol of the rare earth complex shown in formula 1, 10 μmol of [Ph 3 C][B(C 6 f 5 ) 4 ], 100 μmol triisobutylaluminum and chlorobenzene solvent, the concentration of the rare earth complex in the catalyst combination is 2.0mmol L –1 , and reacted for 2 minutes to obtain catalyst combination 1.
[0070] Preparation of catalyst combination 2: at 25°C, add 10 μmol of the rare earth complex shown in formula 2, 10 μmol of [Ph 3 C][B(C 6 f 5 ) 4 ], 100 μmol triisobutylaluminum and chlorobenzene solvent, the concentration of the rare earth complex in the catalyst combination is 0.67mmol L –1 , and reacted for 2 minutes to obtain catalyst combination 2.
[0071] Preparation of Catalyst Combination 3: At 0°C, add 10 μmol of the rare earth complex shown in formula 2, 10 μmol of [Ph 3 C][B(C 6 f 5 ) 4 ], 100 μmol triisobutylaluminum and toluene solvent, the concentratio...
Embodiment 1
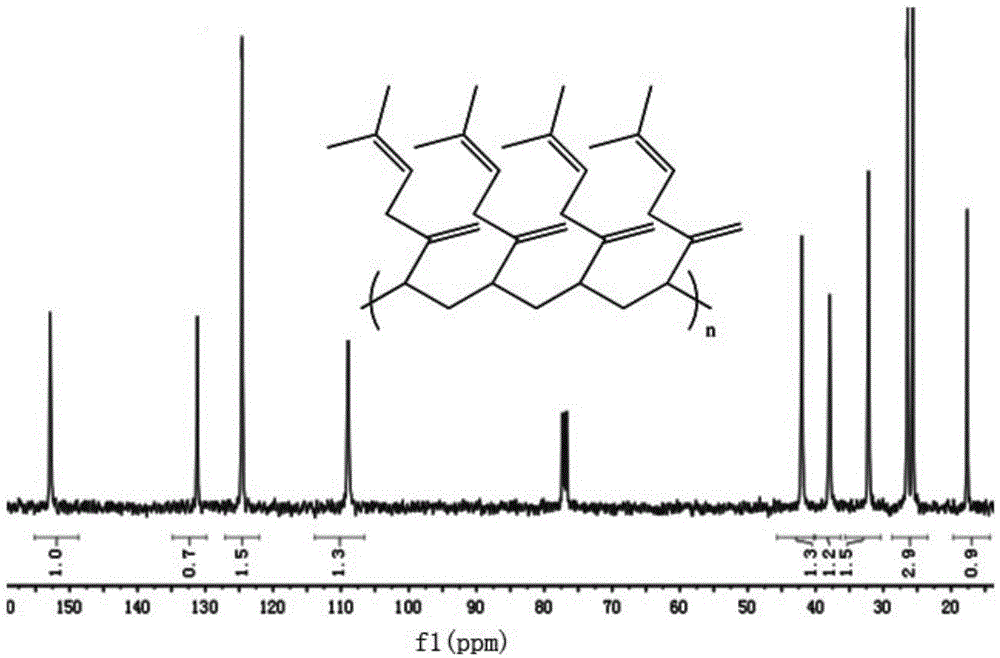
[0091] Take 5ml of the toluene solution of catalyst combination 1, place it in an anhydrous and anaerobic-treated polymerization bottle, add 5.0mmol β-myrcene, carry out the polymerization reaction at 25°C for 0.5 hours, add 2ml of ethanol with a volume concentration of 10% hydrochloric acid The solution terminates the polymerization reaction, and the reaction solution is poured into 100ml of methanol to settle to obtain 3,4-poly-β-myrcene; the obtained polymer is then dried in a vacuum oven for 48 hours to obtain a dry constant weight, with a net weight of 0.68 g. Overall conversion 100%. With NMR spectrum ( 1 H NMR) and carbon nuclear magnetic spectrum ( 13 CNMR) analysis obtains poly-β-myrcene 3, and the content of 4 structural units is 98%; Use GPC analysis to obtain the number-average molecular weight (M) of 3,4-poly-β-myrcene n ) is 134,000, the molecular weight distribution (M w / M n ) is 1.71, and the 3,4-poly-β-myrcene glass transition temperature (T g ) is -42°...
Embodiment 8
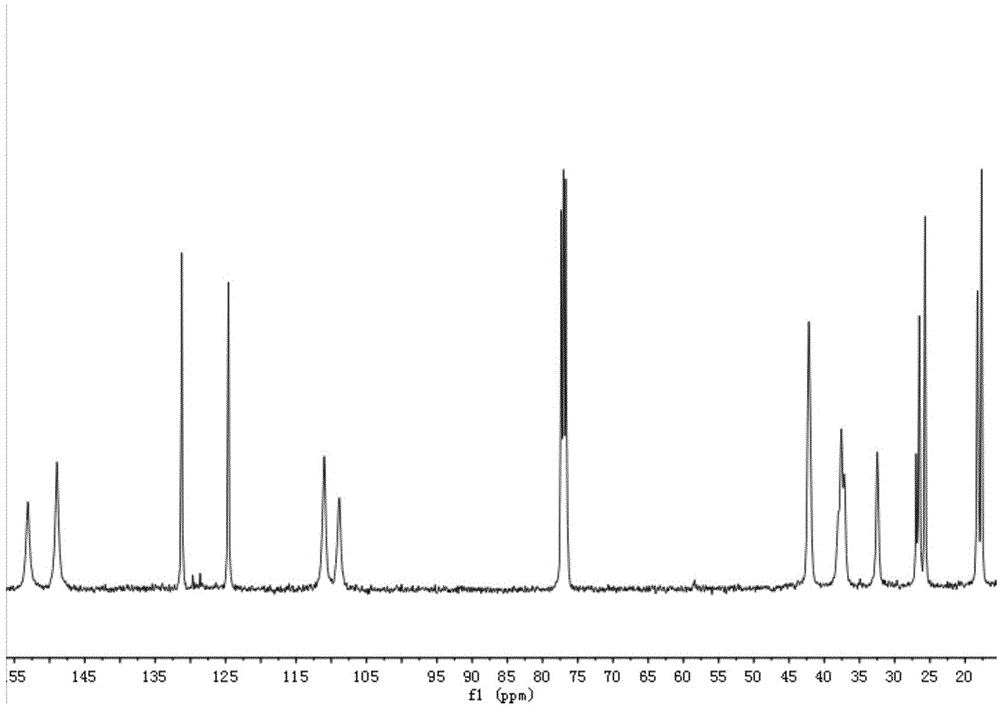
[0094] The solvent used in embodiment 8 is chlorobenzene, figure 1 It is the carbon nuclear magnetic spectrum of the 3,4-poly-β-myrcene prepared in Example 8, and the obtained 3,4-poly-β-myrcene has an isotactic structure mmmm>99%.
[0095] Table 1: 3,4-poly-β-myrcene synthesized in Examples 1-40
[0096]
[0097]
[0098] From the polymerization data of Examples 1 to 40 of the polymerization of 3,4-poly-β-myrcene, it can be concluded that the rare earth catalyst provided by the present invention catalyzes the 3,4 selective polymerization of β-myrcene by means of coordination polymerization , β-myrcene monomer can obtain 100% conversion. The number average molecular weight of 3,4-poly-β-myrcene is 0.1×10 4 ~150×10 4 Within the range, the molecular weight distribution is 1.1-3.0. The rare earth catalyst has high adaptability to temperature, and in the polymerization temperature range of -30-80°C, the 3,4-structure content of poly-β-myrcene can be no less than 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com