Continuous preparation method of 1-acetyl-1-chlorocyclopropane
A technology of chlorocyclopropane and acetyl, which is applied in the field of continuous preparation of 1-acetyl-1-chlorocyclopropane, can solve the problems of long contact time, low production efficiency, and reduced yield, so as to prevent decomposition and deterioration and improve production Efficiency, the effect of simplifying the difficulty of operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
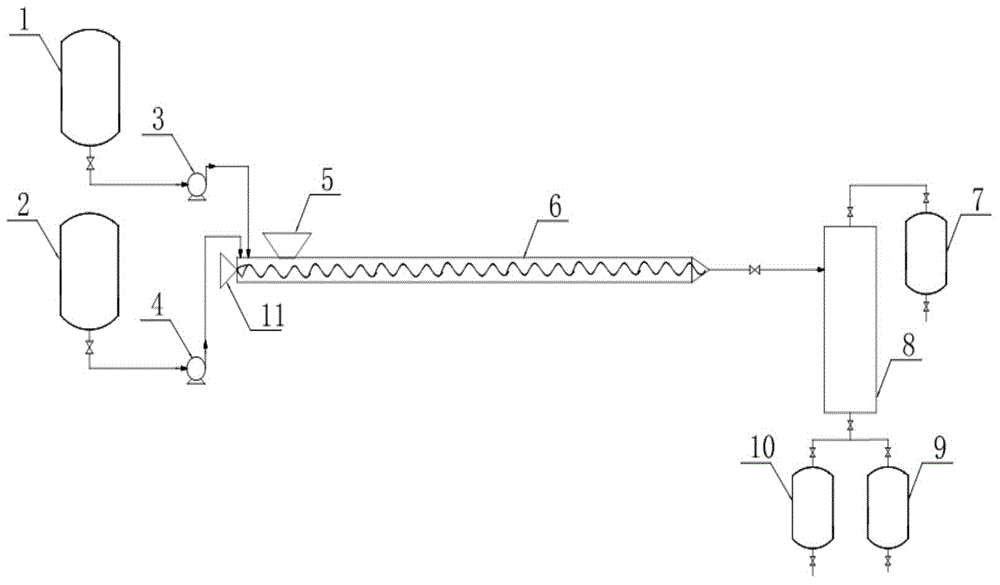
[0019] Step 1) Add 318kg of diethylene glycol and 155kg of 3,5-dichloro-2-pentanone into the preheated reaction kettle respectively, and heat up to 80°C;
[0020] Step 2) Add 58 kg of anhydrous potassium fluoride to the solid feed port; start the agitator of the tubular reactor, adjust the speed to control the material to stay in the reactor for 5 minutes; turn on the heating device and raise the temperature to 130 ° C;
[0021] Step 3) Turn on the metering pump, inject diethylene glycol, 3,5-dichloro-2-pentanone, and potassium fluoride into the tubular reactor in a molar ratio of 3:1:1, and detect at the outlet The content of 3,5-dichloro-2-pentanone≤0.5%; the mixed solution directly enters the thin-film evaporator, the temperature of the evaporator is controlled at 100°C, the vacuum degree is 500Pa, and 126kg of 1-acetyl-1-chlorocyclopropane at the top is collected, Yield 98%, content 96%.
Embodiment 2
[0023] Step 1) Add 318kg of diethylene glycol and 155kg of 3,5-dichloro-2-pentanone into the preheated reaction kettle respectively, and heat up to 50°C;
[0024] Step 2) Add 58kg of anhydrous potassium fluoride to the solid feed port; start the agitator of the tubular reactor, adjust the speed to control the material to stay in the reactor for 1min; turn on the heating device and raise the temperature to 150°C;
[0025] Step 3) Turn on the metering pump, inject diethylene glycol, 3,5-dichloro-2-pentanone, and potassium fluoride into the tubular reactor in a molar ratio of 3:1:1, and detect at the outlet The content of 3,5-dichloro-2-pentanone is ≤0.5%; the mixed liquid enters the thin-film evaporator directly, the temperature of the evaporator is controlled at 80°C, the vacuum degree is 200Pa, and 119kg of 1-acetyl-1-chlorocyclopropane is collected at the top, Yield 93%, content 95%.
Embodiment 3
[0027] Step 1) Add 750kg of triethylene glycol and 155kg of 3,5-dichloro-2-pentanone into the preheated reaction kettle respectively, and heat up to 50°C;
[0028] Step 2) Add 82 kg of anhydrous sodium fluoride to the solid feeding port; start the agitator of the tubular reactor, adjust the speed to control the material to stay in the reactor for 9 minutes; turn on the heating device and raise the temperature to 120 ° C;
[0029] Step 3) Turn on the metering pump, inject triethylene glycol, 3,5-dichloro-2-pentanone, and alkali into the tubular reactor at a molar ratio of 5:1:2, and detect 3, The content of 5-dichloro-2-pentanone≤0.5%; the mixed solution directly enters the thin-film evaporator, the temperature of the evaporator is controlled at 100°C, the vacuum degree is 200Pa, and 105kg of 1-acetyl-1-chlorocyclopropane at the top is collected, and the yield is 82%, content 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com