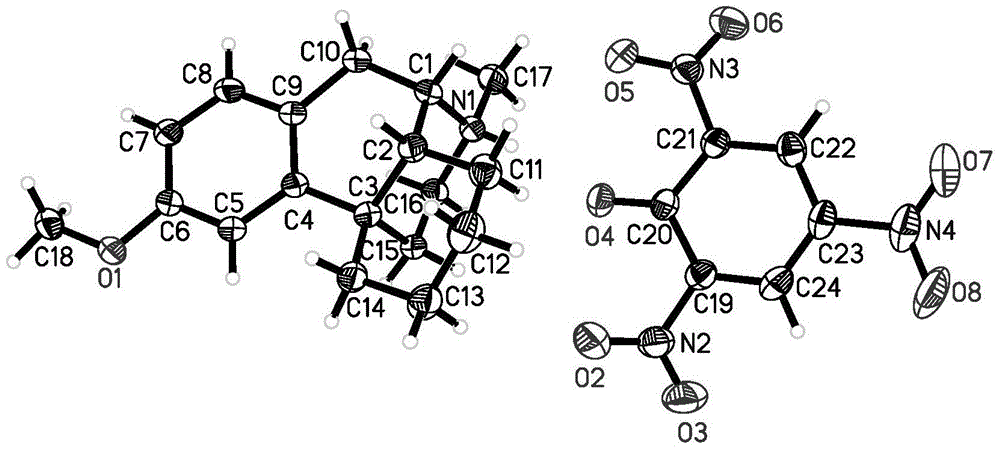
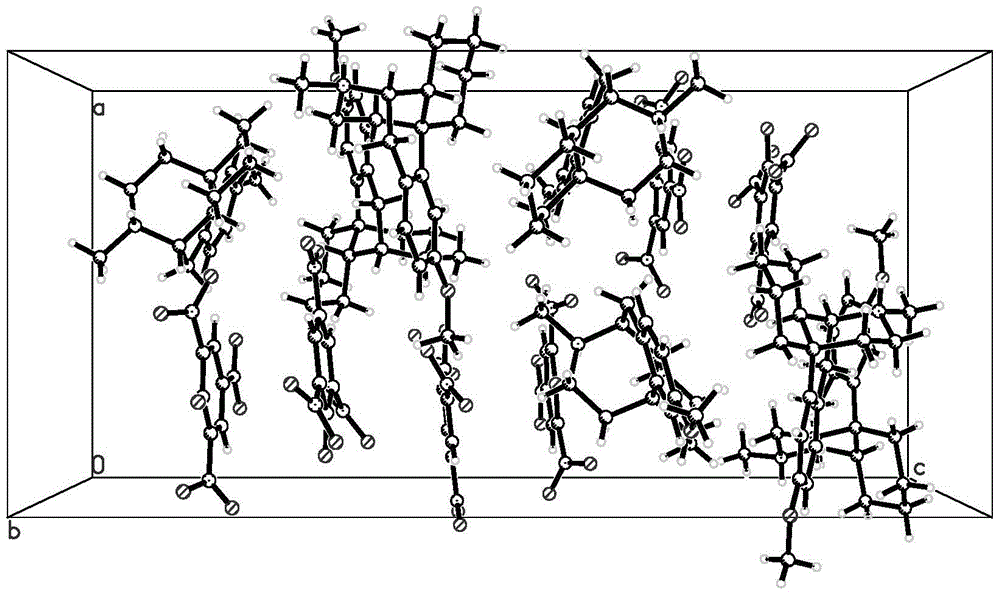
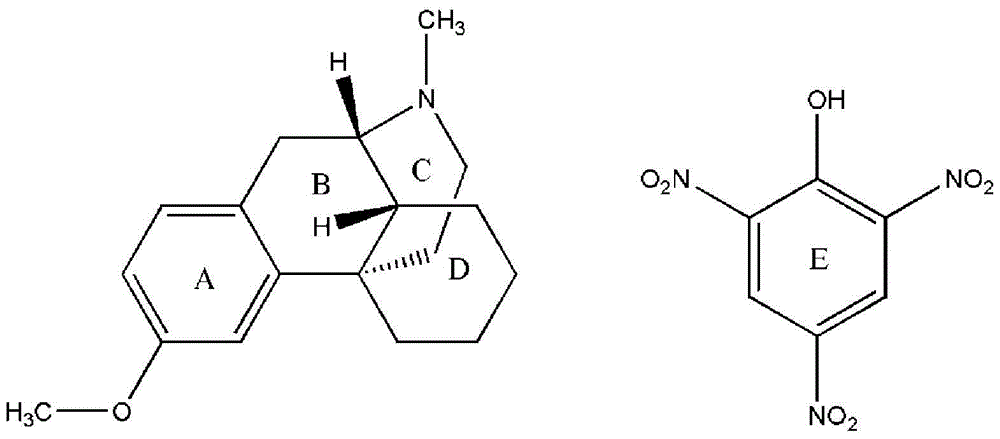
Endo-(14s)-3-methoxy-17-methylmorphinan picrate and its preparation method and use
A technology of picric acid and compounds, applied in the field of compound synthesis, can solve problems such as inconvenient storage, unfavorable detection work, compound instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] During the production process of dextromethorphan, 10.0 g of the mother liquor concentrate of dextromethorphan free base (wherein the HPLC content of the compound of formula B is 6.2%) was added to 25 ml of acetone for recrystallization, and 7.5 g of free base crude product was obtained by filtration, and the mother liquor was concentrated to dryness to obtain 2.4 g of oil (wherein the HPLC content of the compound of formula B is 24.1%). ——This embodiment is to enrich the content of the impurity formula (B) compound in the mother liquor.
Embodiment 2
[0047] Add 10 g of silica gel to the oil obtained in Example 1 and stir evenly, separate and purify by column chromatography, the eluent is (volume ratio): toluene: ethyl acetate: methanol: dichloromethane: ammonia water=55:20:13 :10:2. Sampling was monitored by TLC or HPLC to obtain a solution containing the compound of formula (B) as an impurity, which was concentrated to dryness to obtain 0.2 g of the compound of formula (B) as an oily substance.
[0048] Take 0.2g of the compound of impurity formula (B), dissolve it in 4ml of 95% ethanol, add dropwise a 10% ethanol solution of picric acid, a yellow solid precipitates, and filter until no solid precipitates to obtain a powdery solid, dry to constant Weight, 0.15g of the compound of formula (C) was obtained.
Embodiment 3
[0050] Take 0.15 g of the compound of formula (C) obtained in Example 2, dissolve it in 5 ml of ethanol-acetone (volume ratio 1:1) mixed solution, seal it up but leave gaps, let it stand at room temperature, and cultivate single crystals. Several days later, a single crystal of the compound of formula (C) was obtained, and the absolute stereo configuration was confirmed by single crystal diffraction analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com