A kind of synthetic method of fluorinated benzoheterocyclic-heteroaromatic ring structure
A compound and unsubstituted technology, applied in the field of synthesis of fluorobenzoheterocyclic-heteroaromatic rings, can solve problems such as poor compatibility of functional groups and lengthy reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
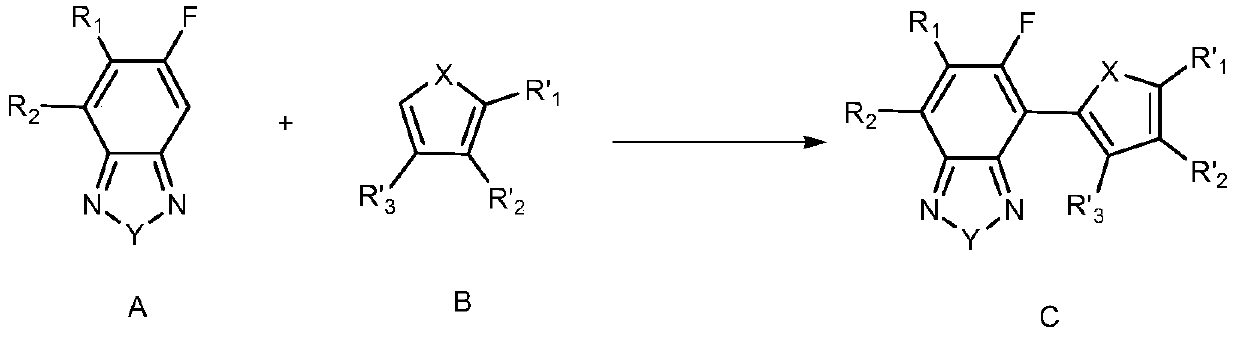
[0074] The invention provides a preparation method of fluorinated benzoheterocyclic-heteroaromatic ring and derivatives thereof. Preferably, the method comprises the steps of:
[0075] In an inert solvent, at a certain temperature (such as 40°C-140°C; preferably 60-100°C), using palladium salt as a catalyst, in the presence of an oxidizing agent, the compound of formula A (i.e. fluorobenzoheterocycle or Its derivative) reacts with the compound of formula B (ie heteroaromatic compound) for a period of time (such as 1-20 hours or 5-10 hours, etc.), thereby forming the compound of formula C (ie fluorobenzoheterocyclic-heteroaryl ring building blocks and their derivatives);
[0076]
[0077] In various forms, R 1 , R 2 , R' 1 , R' 2 , R' 3 , X, Y are defined as mentioned above.
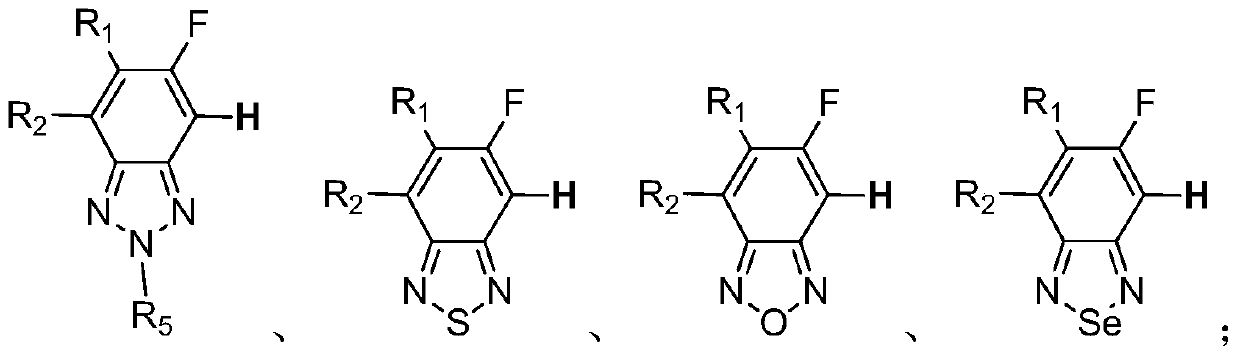
[0078] Wherein, the compound of formula A is a compound selected from the following group:
[0079]
[0080] In various forms, R 1 , R 2 , R 5 as above.
[0081] More preferably, the com...
Embodiment 1
[0122]
[0123] In a three-neck flask, add compound 1 (compound 1 can be prepared with reference to US5514680A1, 5.6g, 25mmol), CHC1 3(150mL), triethylamine (11.4mL, 4equiv). After stirring for 15 minutes, thionyl chloride (SOCl 2 , 7.2g, 2equiv) was slowly dripped into it, and refluxed for 5h. Product 2 (5.1 g, 81% yield) was obtained as a white solid by column chromatography. 1 H NMR (300MHz, CDCl 3 )7.30(t,J=8.1Hz,1H). 19 F NMR (376MHz, CDCl 3 )-120.5(dd,J=19.9Hz,7.5Hz1F),-125.7(dd,J=19.7Hz,8.8Hz1F).
Embodiment 2
[0125]
[0126] 100 mL of H in HOAc (2.4 mL, 40 mmol) 2 O solution, add compound 3 to it, heat to dissolve it completely, after cooling to room temperature, slowly add NaNO 2 (1.52g dissolved in 20mL water) aqueous solution, and then filtered to obtain compound 4. Compound 4 (2.3g, 10mmol) was dissolved in 50mL of methanol, and tBuOK (1.15g, 1.02equiv) and C 8 h 17 Br (1.95g, 1.01 equiv). After reflux and stirring for 24 hours, the column was separated, and the colorless liquid compound 5 (1.20g, 35% yield) was remembered 1 H NMR (400MHz, CDCl 3 )7.55(dd,J=8.6Hz,7.0Hz,1H),4.70(t,J=7.4Hz,2H),2.10(m,2H),1.40-1.15(m,10H),0.86(t,J= 6.8Hz, 3H). 19 F NMR (376MHz, CDCl 3 )-128.9(dd,J=19.2Hz,6.8Hz1F),-129.7(dd,J=19.7Hz,8.8Hz1F).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com