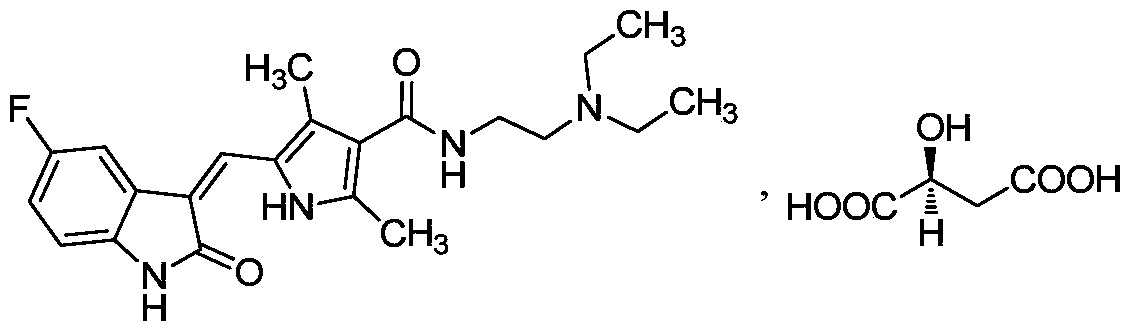
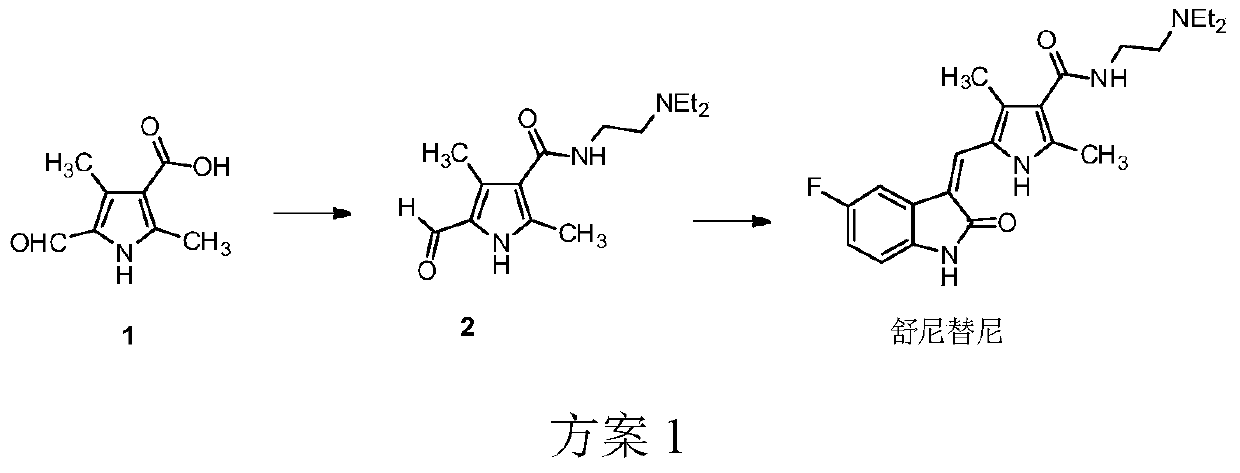
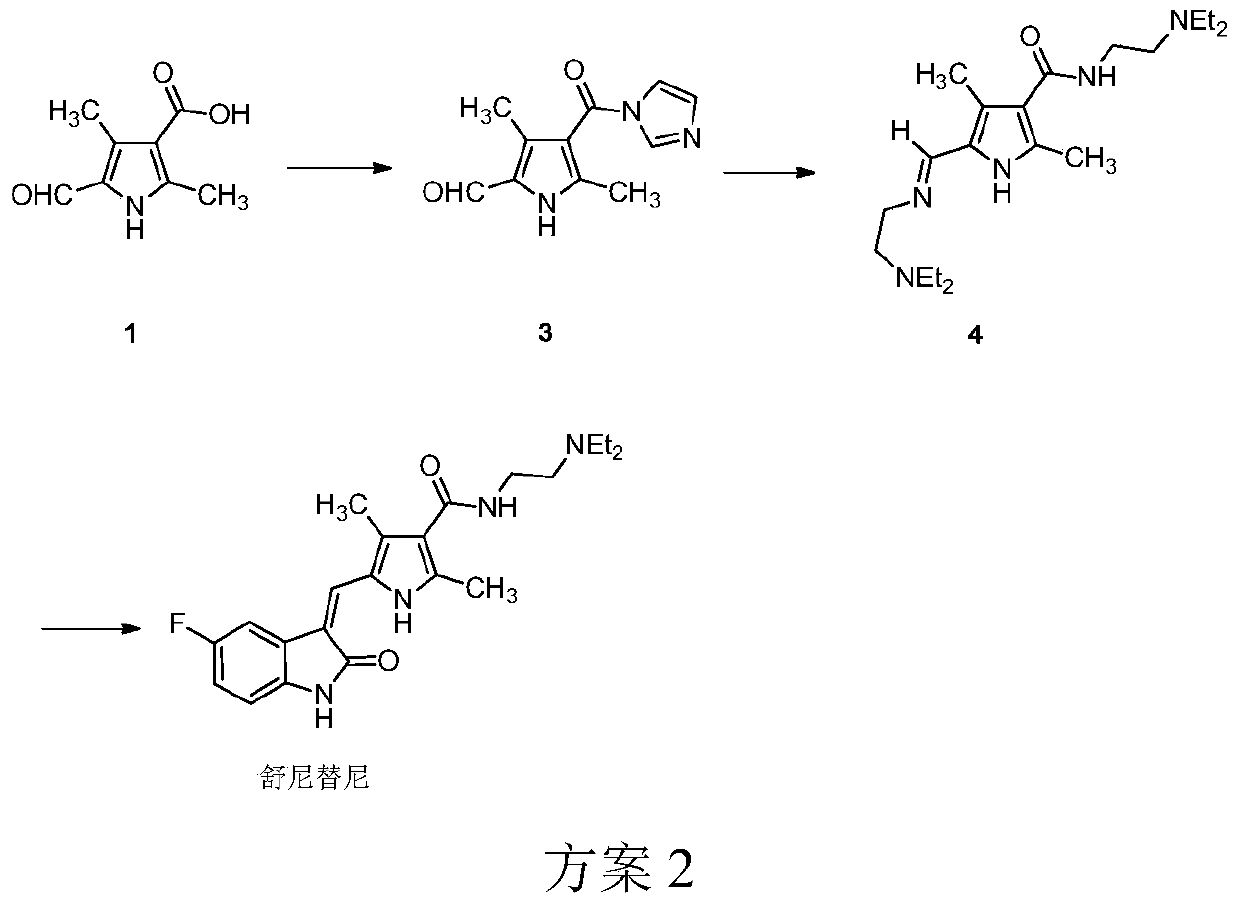
The preparation method of sunitinib malate
A technology of sunitinib malate and malic acid, applied in carboxylate preparation, organic chemistry, etc., can solve the problems of difficult scale-up production, large equipment damage, and affecting product quality, and achieve the goal of reducing impurities and improving purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: (Z)-N-[2-(diethylamino)ethyl]-5-[(5-fluoro-2-oxo-1,2-dihydro-3H-indole-3-ylidene Preparation of base)methyl]-2,4-dimethyl-3-carbamoyl-1H-pyrrole malate
[0035] 2,4-Dimethyl-5-formyl-1H-pyrrole-3-carboxylic acid (200g, 1.2mol, 1eq), carbonyldiimidazole (260g, 1.6mol, 1.3eq) and tetrahydrofuran (4L) were added sequentially In a 10L reaction bottle, control the internal temperature to 35-45°C, and stir for 4 hours. Put the reaction bottle at room temperature, add 1-hydroxybenzotriazole (40g, 0.30mol, 0.25eq), and stir for 1 hour. Cool the reaction to an internal temperature of 10-15°C, slowly add N,N-diethylethylenediamine (460g, 4.0mol, 3.3eq) dropwise to the reaction liquid, control the internal temperature at 10-25°C, and stir for 13 hours . Concentrate the reaction solution to dryness under reduced pressure, add 4 L of ethyl acetate and stir to dissolve, wash four times with 1.5 L of 20% potassium carbonate solution, wash twice with 1.5 L of saturated so...
Embodiment 2
[0041] Example 2: (Z)-N-[2-(diethylamino)ethyl]-5-[(5-fluoro-2-oxo-1,2-dihydro-3H-indole-3-ylidene Preparation of base)methyl]-2,4-dimethyl-3-carbamoyl-1H-pyrrole malate
[0042] 2,4-Dimethyl-5-formyl-1H-pyrrole-3-carboxylic acid (200g, 1.2mol, 1eq), carbonyldiimidazole (260g, 1.6mol, 1.3eq) and toluene (6L) were added sequentially In a 10L reaction bottle, control the internal temperature to 50-60°C, and stir for 3 hours. Put the reaction bottle at room temperature, add 2-hydroxypyridine (14g, 0.15mol, 0.13eq), and stir for 1 hour. Cool the reaction to an internal temperature of 10-15°C, slowly add N,N-diethylethylenediamine (460g, 4.0mol, 3.3eq) dropwise to the reaction liquid, control the internal temperature at 10-25°C, and stir for 15 hours . Then the reaction solution was washed four times with 1.5 L of 20% potassium carbonate solution, washed twice with 1.5 L of saturated sodium chloride solution, dried over anhydrous magnesium sulfate, filtered, and concentrated to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com