RNA-interference-inducing nucleic acid molecule able to penetrate into cells, and use therefor
A technology of nucleic acid molecules and penetrating ability, applied in DNA/RNA fragments, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of induced toxicity, weakened siRNA delivery efficiency, inability to deliver siRNA efficiently, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
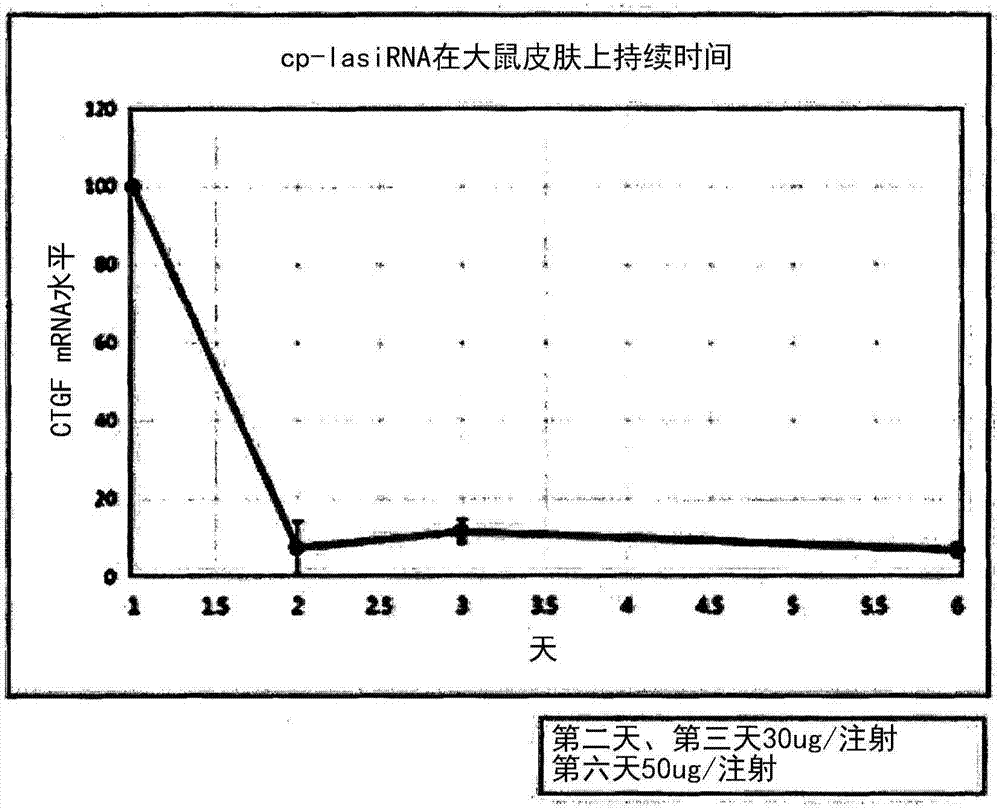
[0077] Example 1: Screening of RNAi-inducing double-stranded nucleic acid molecules using CTGF as a target
[0078] Before introducing various chemical modifications for an effective self-delivery structure, after designing 50 kinds of target sequences for CTGF in order to ensure a highly efficient RNAi-inducing double-stranded nucleic acid molecule targeting CTGF Perform screening.
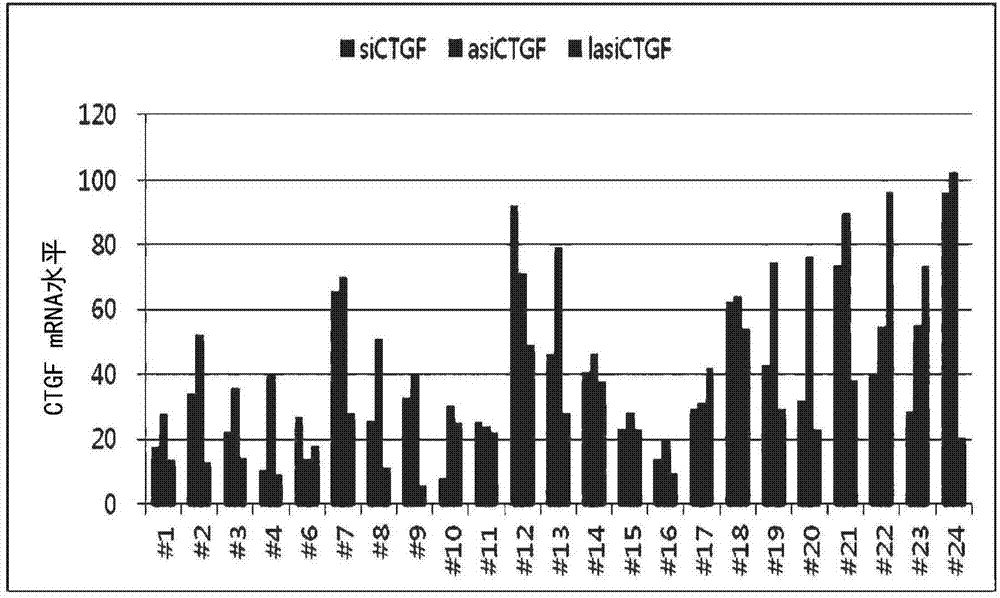
[0079] In order to compare the CTGF gene suppression efficiency of lasiRNA and conventional RNAi-inducing constructs, siRNA, asiRNA, and lasiRNA constructs targeting each nucleotide sequence were synthesized as shown in Tables 1 to 3. Tables 1 to 3 are the base sequence information of siRNA, asiRNA, and lasiRNA structures of 24 sequences targeting CTGF (large text: RNA, small text: DNA). In order to test (test) the CTGF mRNA expression inhibitory effect of each base sequence and structure, each structure was transfected (transfection) with HaCaT (ATCC) at 10 nM, and the CTGF mRNA expression leve...
Embodiment 2
[0106] Example 2: Production of Nucleic Acid Molecules of the Present Invention and Measurement of Intracellular Absorption Rate
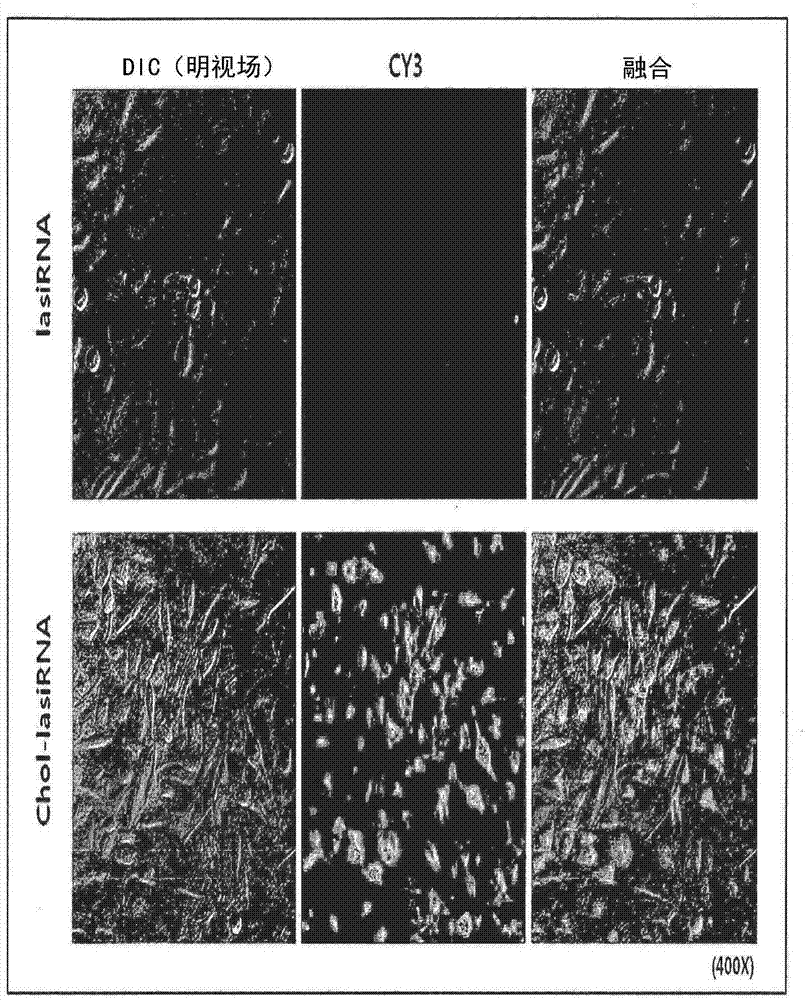
[0107] 2-1: Effect of cholesterol modification
[0108] First, in order to confirm the impact of cholesterol modification on the delivery of lasiRNA, the sense strand of lasiRNA, that is, the 5' end of the second strand, is labeled with cy3, and the presence or absence of cholesterol is determined with a fluorescence microscope. The resulting uptake difference. That is, the cy3-labeled lasiRNA or chol-lasiRNA structure was incubated (incubated) at 1uM in HeLa cells, and observed with a fluorescence microscope after 3 hours to compare the degree of intracellular transmission.
[0109] First, in a 100mm Petri dish (Petri dish) supplemented with 10% fetal bovine serum (Gibco company), 100 μg / ml penicillin (penicillin) / streptomycin (streptomycin) Dulbecco modified Eagle HeLa cells (ATCC) were cultured in Dulbecco's modified Eagle's medium (Gibco). ...
Embodiment 3
[0119] Example 3: Determination of CTGF Expression Inhibition Efficiency
[0120] Using the results of the internalization test of Cy3-labeled lasiRNA in Example 2, it can be confirmed that by directly introducing cholesterol (Cholesterol) and PS modification (PS modification) into the lasiRNA structure, no delivery vehicle or additional Reagents that also enable efficient intracellular delivery of lasiRNA. However, when various chemical modifications are introduced into siRNA, it is known that the activity of the siRNA is reduced to some extent or the activity of the siRNA is drastically reduced depending on the modification. After transfection (transfection) of lasiRNA with various structures into HeLa cells, the expression (expression) change of CTGF mRNA was measured, thereby determining the influence of each modification (modification) on the gene expression inhibition of lasiRNA.
[0121] In order to confirm the effect of PS modification (PS modification) on the gene in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com