A method of continuously preparing 3,5,5-trimethyl-3-cyclohexene-1-one
The technology of a trimethylcyclohexyl and reactive distillation column is applied in the field of continuous preparation of 3,5,5-trimethylcyclohex-3-en-1-one, and can solve the problems of low extraction rate, difficulty in industrial production, The problems of low space-time yield and other problems have achieved the effects of easy recovery, less equipment corrosion, and less generation of three wastes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
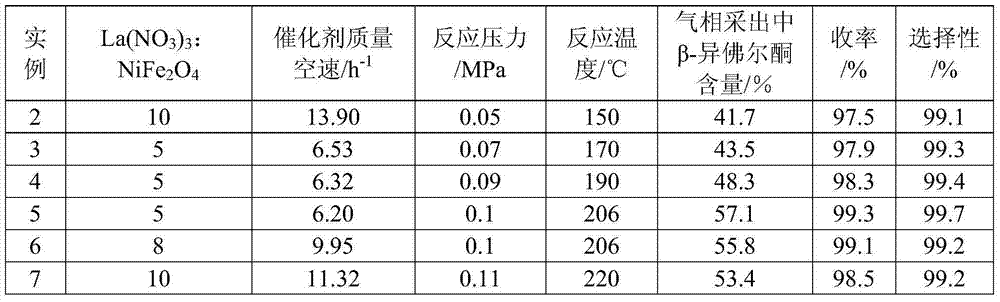
[0036] 10gLa(NO 3 ) 3 Dissolve in 200ml deionized water to form a salt solution, and La(NO 3 ) 3 : NiFe 2 o 4 (Zibo Innaite Nano Technology Co., Ltd.) = 15:1 (mass ratio), with an appropriate amount of NiFe 2 o 4Add it to the salt solution, stir well, and soak for 8 hours. After filtering, dry at 60°C for 8h; then bake at 600°C for 3h in a muffle furnace to obtain the magnetic nano-solid alkali catalyst La 2 o 3 / NiFe 2 o 4 .
[0037] Add 10g La in advance at the bottom of 1m rectification tower 2 o 3 / NiFe 2 o 4 and 200g alpha-isophorone. The reaction system was replaced by nitrogen for 3 times. Under the conditions of 206°C and 0.1MPa, the number of plates was 20, and the reflux ratio was 3. After 1 hour of stable total reflux, the top and bottom of the tower began to be withdrawn, and the bottom of the tower began to feed α - Isophorone, the flow rate is 100g / h for continuous reactive distillation. After 48 hours, the selectivity of β-isophorone measured by...
Embodiment 8
[0041] With reference to the technical process of Example 1, only the continuous reaction rectification time is extended at 500h, the reaction selectivity is maintained at 99.5%, and the total yield is 98.1%. After 1000 hours of continuous reaction and rectification, the activity of the catalyst decreased. The catalyst was recovered by applying an external magnetic field, and the activity recovered after being calcined again in a muffle furnace at 600°C for 3 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com