Near-infrared absorbing phosphorescent material based on rhodium tetraphenylporphyrin-azafluoroboron dipyrrole and its preparation method and application
A technology of heterofluoroboron dipyrrole and rhodium tetraphenylporphyrin, which is applied in the field of near-infrared absorbing phosphorescent materials, can solve problems such as short luminescence life, and achieve the effect of long phosphorescence decay life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthesis of embodiment 1. compound A:
[0028] Add rhodium tetraphenyl porphyrin chloride Rh (ttp) Cl (15.8 mg, 0.019 mmol), bromo azafluoro boron dipyrrole aza-BODIPY-a (13.7 mg, 0.021 mmol) in the reactor, K 2 CO 3 (53.9mg, 0.39mmol) and 1.0mL of benzene, the reaction mixture was refrigerated and degassed three times, and reacted at 150°C for 28 hours under the protection of nitrogen. After the reaction is finished, the solvent is spin-dried under reduced pressure, and the 2 Cl 2 / Hexane (1:1) developer was separated by silica gel column chromatography to obtain 17.1 mg of compound A. Yield: 61%.R f =0.61 (CH 2 Cl 2 / hexane=1:1). 1 H NMR (400MHz, CDCl 3 ):δ0.44(d,J=8.5Hz,2H),2.69(s,12H),5.45(d,J=8.4Hz,2H),5.98(s,1H),6.75(s,1H),7.30 -7.36(m,6H),7.45(d,J=8.0Hz,2H),7.51-7.52(m,9H),7.56-7.63(m,3H),7.80(d,J=3.3Hz,2H), 8.04(d, J=7.7Hz, 4H), 8.09(d, J=7.8Hz, 4H), 8.87(s, 8H).; HRMS(FABMS): Calcd for [C 80 h 56 BBrF 2 RhN 7 ] + ([M] + ): m / z 1347.2903. Fou...
Embodiment 2
[0029] Embodiment 2. Synthesis of compound B:
[0030] Add rhodium tetraphenylporphyrin chloride Rh (ttp) Cl (15.8 mg, 0.019 mmol), bromo azafluoro boron dipyrrole aza-BODIPY-b (13.7 mg, 0.021 mmol) in the reactor, K 2 CO 3 (53.9mg, 0.39mmol) and 1.0mL of benzene, the reaction mixture was refrigerated and degassed three times, and reacted at 150°C for 30 hours under the protection of nitrogen. After the reaction is finished, the solvent is spin-dried under reduced pressure, and the 2 Cl 2 / Hexane (1:1) developer was separated by silica gel column chromatography to obtain 11.4 mg of compound B. Yield: 66%.R f =0.57(CH 2 Cl 2 / hexane=1:1). 1 H NMR (400MHz, CDCl 3 ):δ0.52(d,J=8.6Hz,2H),2.71(s,12H),5.57(d,J=9.0Hz,2H),6.22(s,1H),6.71(s,1H),7.25 -7.30(m,3H),7.48(d,J=7.8Hz,2H),7.51-7.58(m,9H),7.60-7.67(m,6H),7.82(d,J=7.2Hz,2H), 8.06(d, J=7.6Hz, 8H), 8.83(s, 8H).HRMS(FABMS): Calcd for [C 80 h 56 BBrF 2 RhN 7 ] + ([M] + ):m / z1347.2903.Found:m / z.1347.2908.
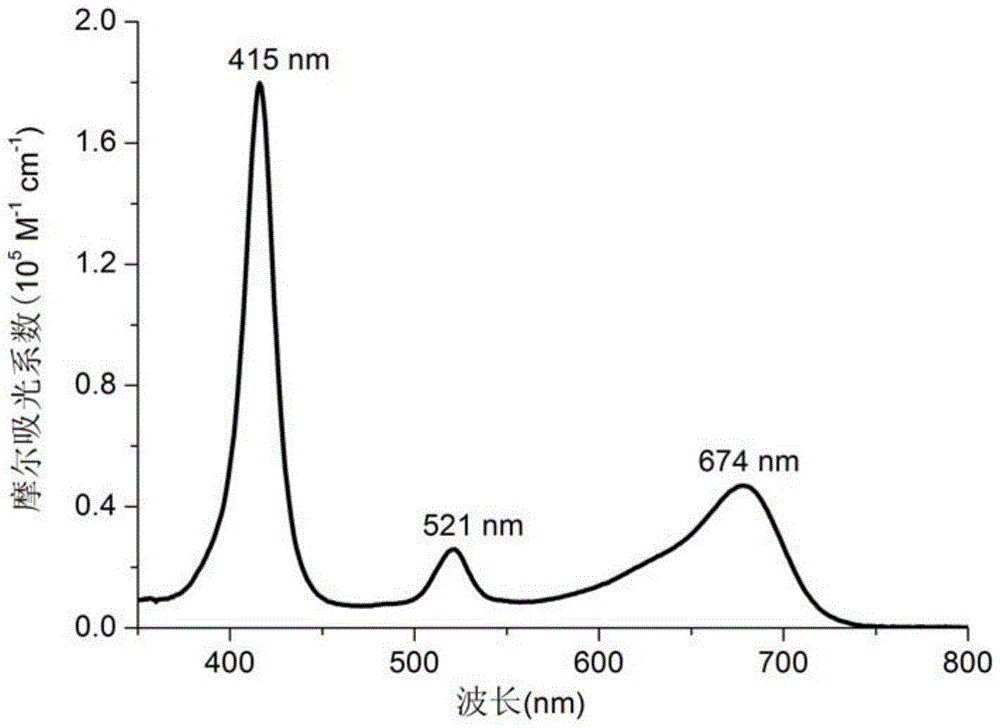
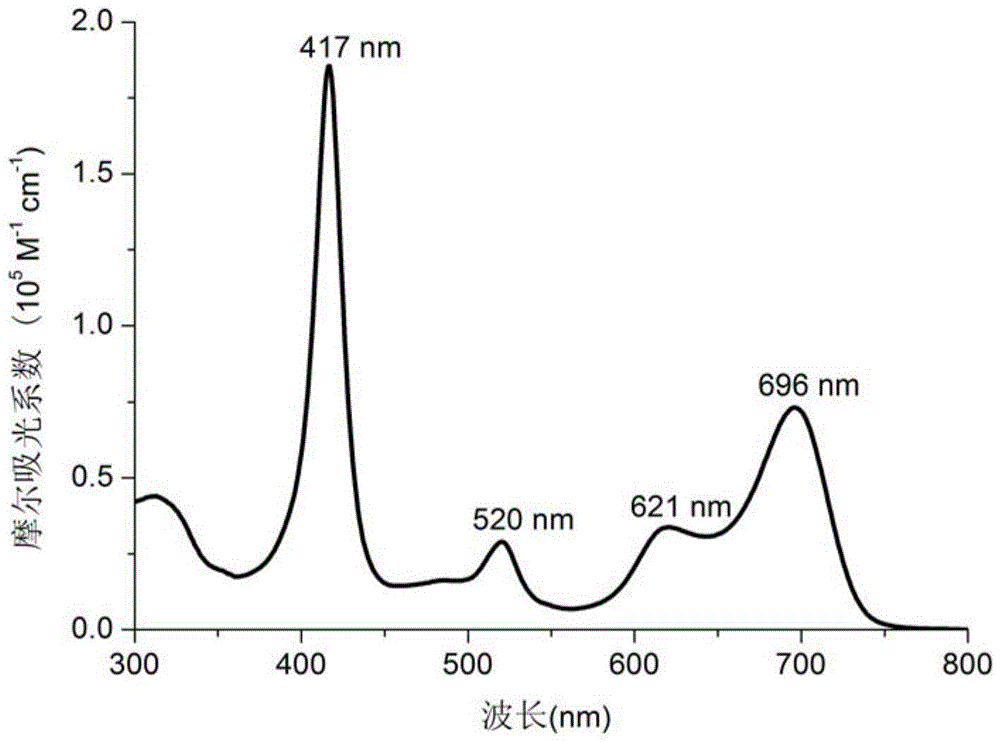
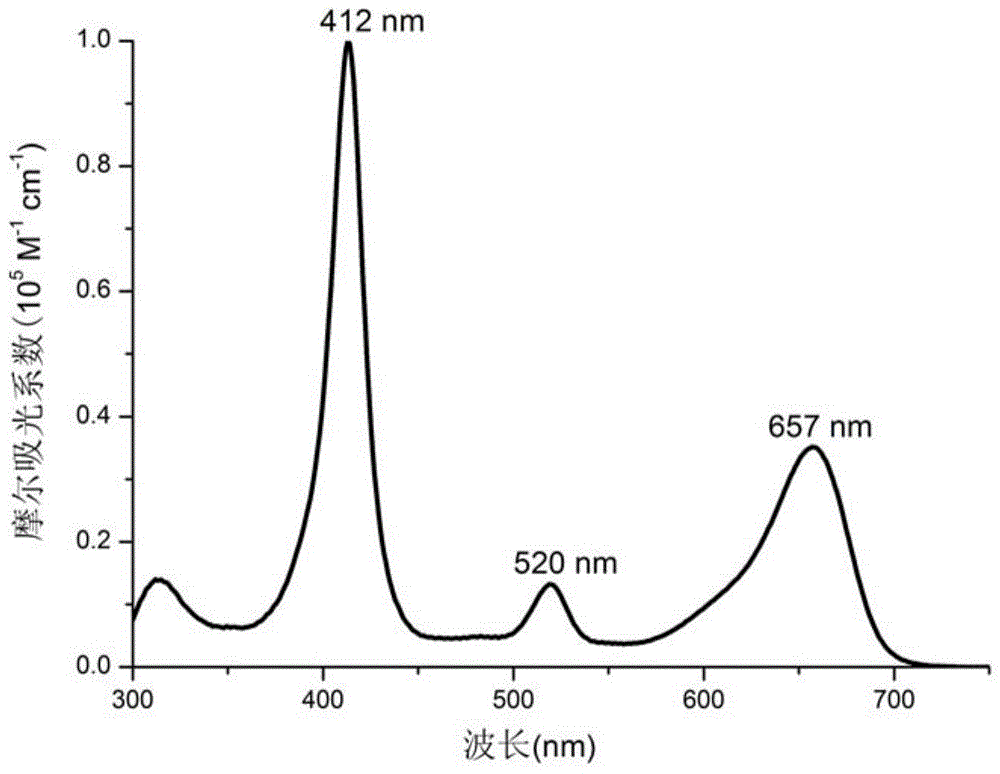
[0031] Its U...
Embodiment 3
[0032] Embodiment 3. Synthesis of compound C:
[0033] Add rhodium tetraphenylporphyrin chloride Rh (ttp) Cl (15.8mg, 0.019mmol), bromoazine fluoroboron dipyrrole aza-BODIPY-c (13.7mg, 0.021mmol), K 2 CO 3 (53.9mg, 0.39mmol) and 1.0mL of benzene, the reaction mixture was refrigerated and degassed three times, and reacted at 150°C for 32 hours under the protection of nitrogen. After the reaction is finished, the solvent is spin-dried under reduced pressure, and the 2 Cl 2 The developer of / hexane (1:1) was separated by silica gel column chromatography to obtain 6.74 mg of compound B. Yield: 39%. 1 HNMR (400MHz, CDCl 3 ):δ0.40(d,J=8.4Hz,1H),0.44(s,1H),2.67(s,12H),5.00(t,J 1 =4.0Hz,J 2 =7.8Hz,1H),5.51(s,1H),6.33(d,J=7.4Hz,1H),6.69(s,1H),6.88(t,J 1 =7.8Hz,J 2 =7.8Hz,1H),7.17(d,J=7.8Hz,1H),7.37-7.39(m,8H),7.47-7.50(m,2H),7.53-7.55(m,5H),7.71(s, 1H), 7.81-7.82(m, 4H), 7.84(d, J=8.0Hz, 4H), 7.99(d, J=7.4Hz, 4H), 8.08(s, 8H).HRMS(FABMS): Calcd for [C 80 h 56 BBrF 2 RhN 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com