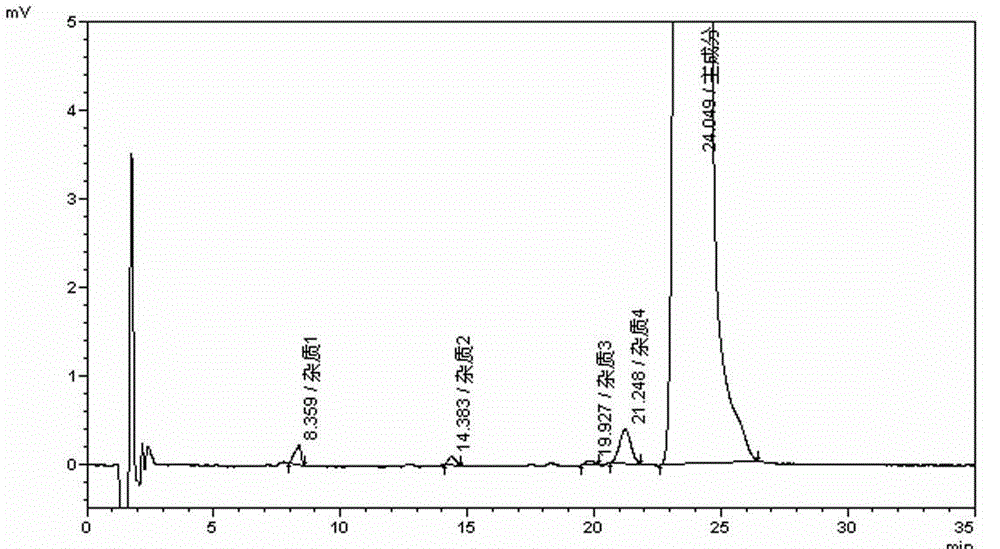
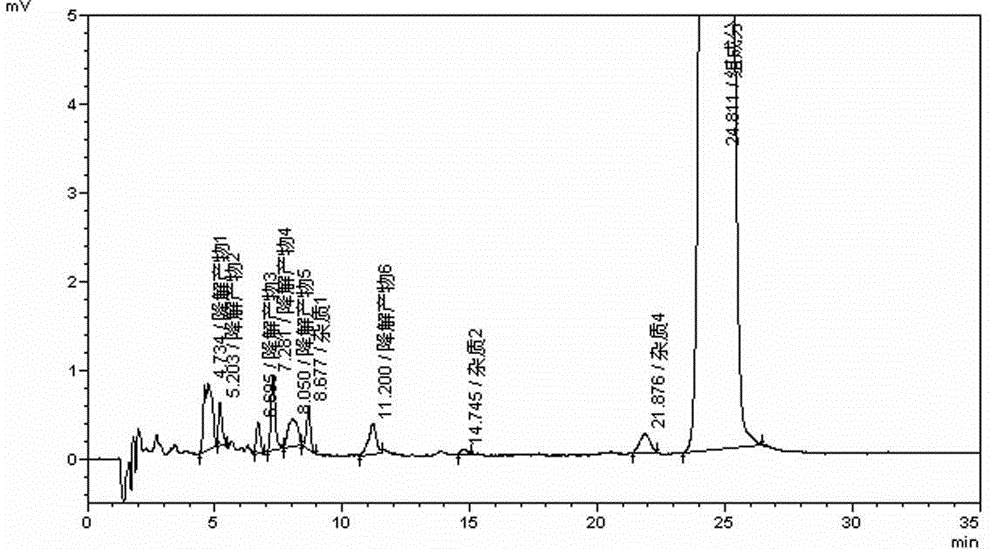
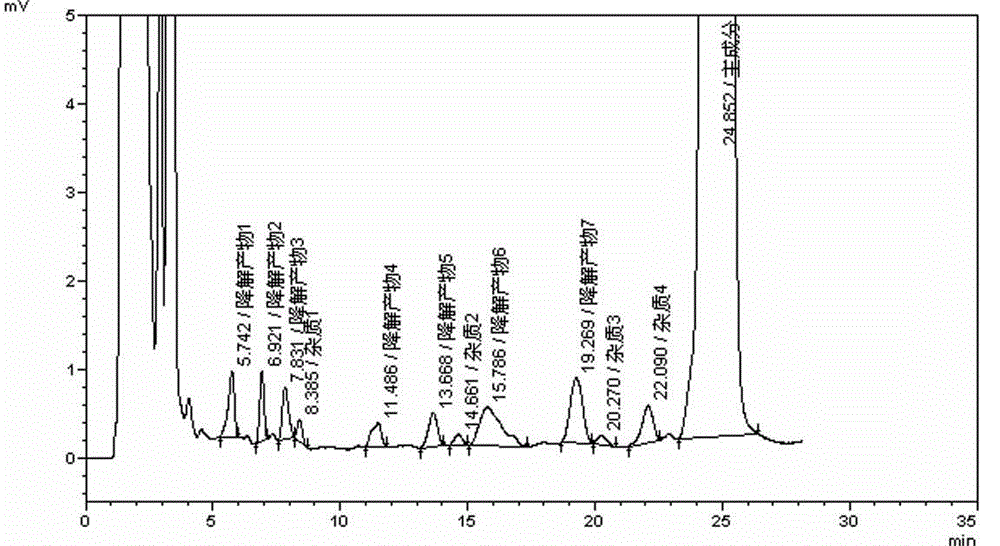
Desloratadine syrup preparation and preparation method thereof
The technology of desloratadine and loratadine is applied in the field of syrup medicament and its preparation, which can solve the problems of unsolved taste problem, undisclosed sucrose-containing, large sucrose, etc. The effect of improving and inhibiting degradation of degraded impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] A preparation method of a syrup preparation of desloratadine as described above, said preparation method comprising the steps of:
[0055] (1) Hypromellose is dissolved with an appropriate amount of water to make a certain concentration;
[0056] (2) Desloratadine is dissolved in propylene glycol;
[0057] (3) Dissolve the pH regulator, stabilizer, and preservative with an appropriate amount of water, and then add sucrose to dissolve;
[0058] (4) Slowly add step (2) to step (3) while stirring to make the solution clear, then add the solution of step (1) and flavoring agent, stir evenly, filter and add water to the full amount, and sterilize at 60°C 6 hours.
[0059] The preferred scheme of the present invention is: in the described step:
[0060] In step (1), hypromellose is added with hot water at 70-80°C under stirring, and stirred for several minutes until it is uniformly dispersed, then an appropriate amount of water is added, and stirred to dissolve it;
[006...
Embodiment
[0074] Embodiment: The multiple combinations of stabilizers described in the present invention include mixtures of antioxidants, hypromellose, propylene glycol, glycerin, and water-soluble beta-cyclodextrin.
[0075] The preparation method of desloratadine sucrose of the present invention is:
[0076] (1) Hypromellose is dissolved with an appropriate amount of water to make a certain concentration;
[0077] (2) Desloratadine is dissolved in propylene glycol;
[0078] (3) Dissolve the pH regulator, stabilizer, and preservative with an appropriate amount of water, and then add sucrose to dissolve;
[0079] (4) Slowly add step (2) to step (3) while stirring to make the solution clear, then add the solution of step (1) and flavoring agent, stir evenly, filter and add water to the full amount, and sterilize at 60°C 6 hours.
Embodiment 1
[0103] prescription composition
[0104]
[0105] Preparation method:
[0106] (1) Add hypromellose with hot water at 70-80°C under stirring, stir for a few minutes to disperse evenly, then add appropriate amount of water, stir to dissolve;
[0107] (2) Desloratadine is dissolved in propylene glycol;
[0108] (3) Add citric acid, sodium citrate, EDTA-2Na, sodium benzoate, and hydroxypropyl-β-cyclodextrin into an appropriate amount of water, stir to dissolve, add sucrose and stir to dissolve;
[0109] (4) Slowly add steps (1) and (2) to step (3) while stirring to clarify the solution, then add menthol and cooling agent 3, stir evenly, filter and add water to the full amount. Sterilize at 60°C for 6 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com