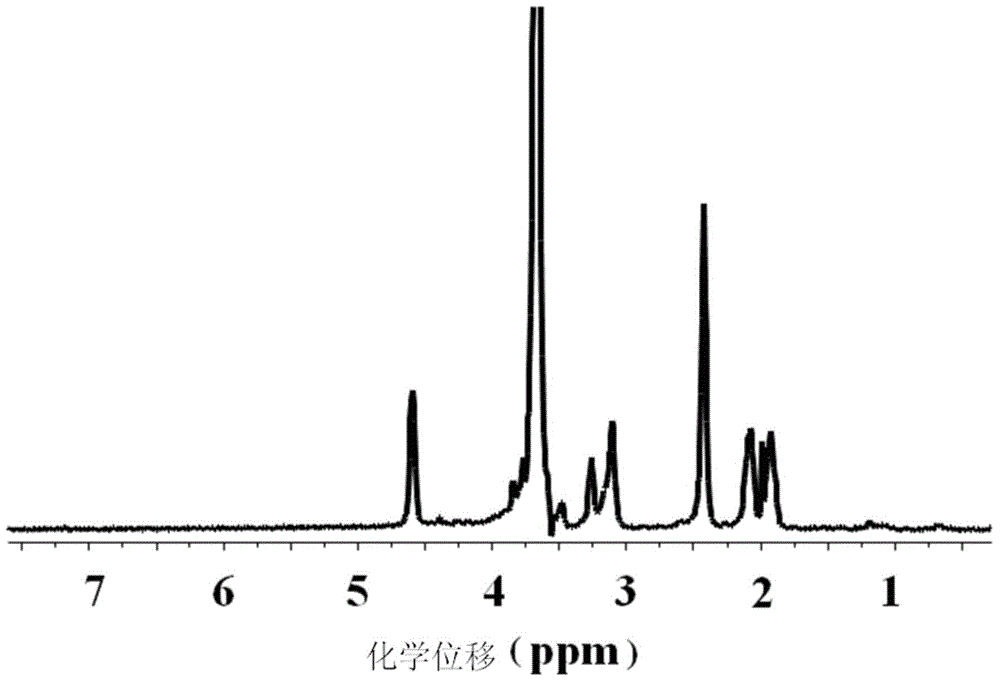
Medicine co-carried compound, micelle and preparation method of micelle
A compound and co-loading technology, which can be used in drug combinations, pharmaceutical formulations, medical preparations with inactive ingredients, etc., can solve problems such as the inability to meet the needs of the market, achieve good tumor growth, good stability, and reduce toxic and side effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
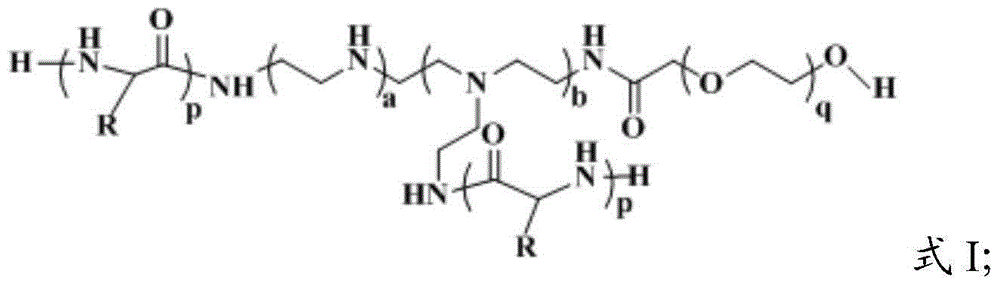
[0048] The present invention has no special limitation on the preparation method of the multi-arm copolymer having the structure of formula I, and the technical solution for preparing the multi-arm copolymer having the structure of formula I that is well known to those skilled in the art can be used. In the present invention, the preparation method of the multi-arm copolymer having the structure of formula I preferably comprises the following steps:
[0049] Mixing and reacting polyethyleneimine, amine-based polyethylene glycol derivatives, and amino acid N-carboxylic acid anhydride in the ring, and deprotecting to obtain a multi-arm copolymer;
[0050] The amino acid N-carboxyl ring acid anhydride is glutamic acid N-carboxyl ring acid anhydride or aspartic acid N-carboxyl ring acid anhydride;
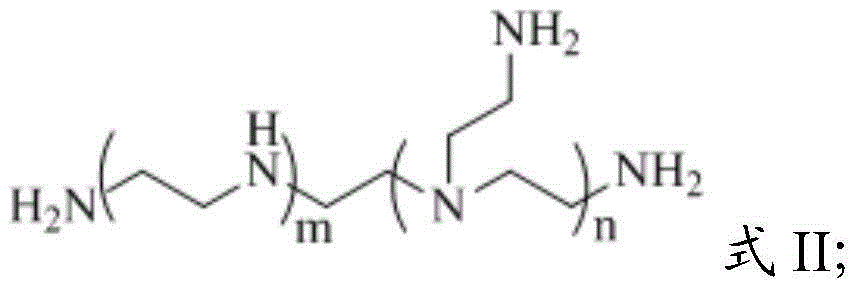
[0051] The polyethyleneimine has the structure of formula II;
[0052]
[0053] m and n are degrees of polymerization; the number average molecular weight of the polyethyleneimine ...
Embodiment 1
[0084] Add 5.0 g of mPEG-NHS with a number average molecular weight of 2000 Da to the dry reaction flask, add 50 mL of anhydrous N,N-dimethylformamide to dissolve, add 1.5 g of polyethyleneimine with a molecular weight of 600 Da, and stir at room temperature for 24 h. Dialyze with deionized water for 72 hours in a 7000Da dialysis bag, change the water 6-8 times, and freeze-dry to obtain mPEG-PEI;
[0085] Weigh 2.6g of mPEG-PEI, use toluene to azeotropically remove water for 3h, vacuum dry, add 60mL of anhydrous N,N-dimethylformamide to dissolve, weigh 10.48g of glutamic acid NCA to another dry reaction safe Vacuum the bottle, add 60 mL of anhydrous DMF to dissolve with a syringe, quickly add mPEG-PEI to glutamic acid NCA, and react at room temperature for 72 hours. The product was settled with more than 10 times the volume of anhydrous ether, filtered, washed and dried to obtain a compound with a protective group.
[0086]Take 2 g of the compound with a protective group, dis...
Embodiment 2
[0089] Add 5.0 g of mPEG-NHS with a number average molecular weight of 5000 Da to the dry reaction flask, add 50 mL of anhydrous N,N-dimethylformamide to dissolve, add 0.6 g of polyethyleneimine with a molecular weight of 600 Da, and stir at room temperature for 24 h. Dialyze with deionized water for 72 hours in a 7000Da dialysis bag, change the water 6-8 times, and freeze-dry to obtain mPEG-PEI;
[0090] Weigh 2.8g of mPEG-PEI, use toluene to azeotropically remove water for 3h, vacuum dry, add 60mL of anhydrous N,N-dimethylformamide to dissolve, weigh 5.24g of glutamic acid NCA to another dry reaction safe Vacuum the bottle, add 30 mL of anhydrous DMF to dissolve with a syringe, quickly add mPEG-PEI to glutamic acid NCA, and react at room temperature for 72 hours. The product was settled with more than 10 times the volume of anhydrous ether, filtered, washed and dried to obtain a compound with a protective group.
[0091] Take 2 g of the compound with a protective group, dis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com