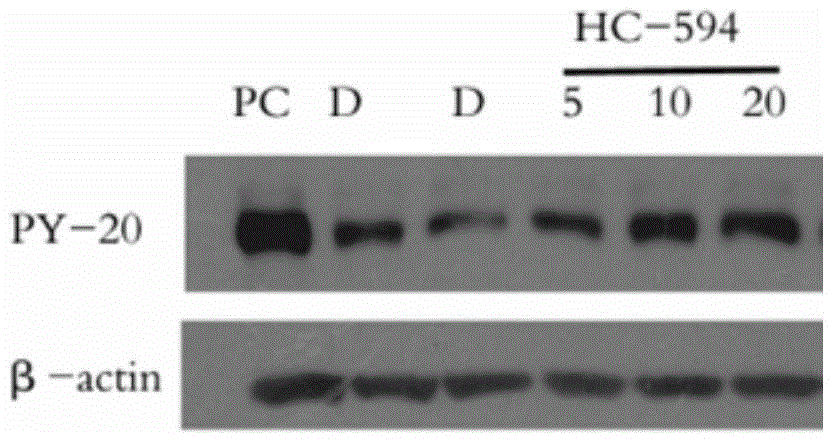
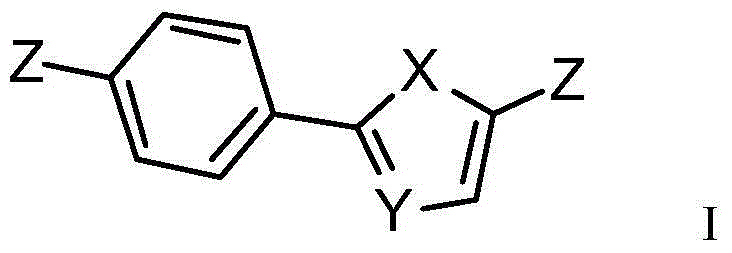
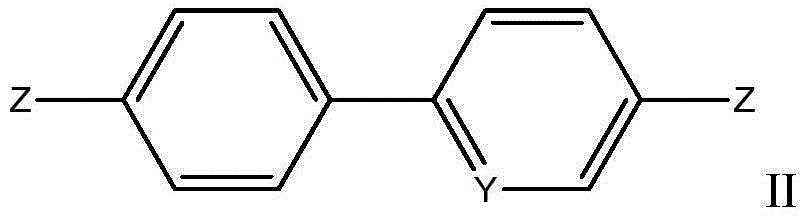
Phenyl ring-aromatic ring cascaded compound, and preparation method and medical application thereof
A compound and aryl technology, applied in the field of medical technology, can solve problems such as poor cell permeability, low bioavailability, and difficulty in making medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119]
[0120] Reagents and conditions: a) Benzoyl chloride, pyridine, DCM
[0121] Compound 1-1 (552 mg, 3 mmol) and pyridine (4.5 mL, 18 mmol) were dissolved in DCM (10 mL), and benzoyl chloride (833 μL, 7.2 mmol) was added under ice-cooling conditions. After the reaction was detected by TLC, the reaction solution was filtered, and the solid was washed with methanol and water, and dried to obtain compound 3-2 (3-HC-392) (700 mg, 60%). 1 H NMR(400MHz,DMSO-d6)δ7.57(t,J=7.4Hz,4H),7.63(t,J=7.0Hz,2H),7.70(d,J=8.8Hz,4H),7.90(d ,J=8.4Hz,4H),7.99(d,J=7.6Hz,4H),10.35(s,2H).
[0122] Except that benzoyl chloride is appropriately replaced by the corresponding reaction compound, the preparation of the following compounds can refer to the preparation method in Example 1.
[0123]
Embodiment 2
[0125]
[0126] Reagents and conditions: a) piperidine, 57% perchloric acid; b) fuming nitric acid, acetic anhydride, 0°C; c) p-nitroacetophenone, triethylamine, acetic acid, ammonium acetate, acetonitrile, 0°C, room temperature; d) hydrazine hydrate, palladium carbon, THF; e) benzoyl chloride, triethylamine, THF
[0127] Compound 4 (98mL, 595mmol) and 57% perchloric acid (56mL) were stirred at room temperature for 1.5h, then a solution of piperidine (117.6mL, 1190mmol) was added dropwise under ice-cooling conditions, and after the addition was completed, it was stirred at 0°C for 40min , and then add 57% perchloric acid 70mL. After the reaction was completed, the reaction solution was placed in a refrigerator at 4 °C for 3 h, and the precipitated solid was filtered, washed with ether, and dried to obtain compound 5 (52.48 g, 29%). 1 H NMR (400MHz, DMSO-d 6 )δ1.50-1.70(m,12H),3.45-3.70(m,8H),5.82(t,J=11.8Hz,1H),7.69(d,J=12.0Hz,2H).
[0128] Compound 5 (50g, 163mmol) was ...
Embodiment 3
[0136]
[0137] Reagents and conditions: a) Boc anhydride, K 2 CO 3 ,THF,H 2 O; b) Et 3 N, HATU, DCM, 40°C; c) trifluoroacetic acid, CH 2 Cl 2 ,40℃; d) Benzoyl chloride, triethylamine, DCM
[0138] Compound 10 (10 g, 77.5 mmol) and potassium carbonate (21.4 g, 155 mmol) were dissolved in water (150 mL), and a solution of Boc anhydride (16.9 g, 77.5 mmol) in THF (50 mL) was slowly added dropwise under ice cooling. After the dropwise addition, it was reacted at room temperature for 12 hours. After the reaction was completed, THF was removed by rotary evaporation, and the pH was adjusted to 1 with 1N hydrochloric acid. A large amount of solid precipitated, filtered, and the obtained solid was washed once with water, and dried to obtain compound 11 (30.4 g, 86%). 1 H NMR (400MHz, CDCl 3 )δ1.45(s,9H),1.59-1.69(m,2H),1.89-1.92(m,2H),2.45-2.52(m,1H),2.82-2.88(m,2H),3.95-4.10( m,2H).
[0139] Compound 11 (11g, 48mmol) was added to DCM (100mL), benzidine (3.68g, 20mmol), tri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com