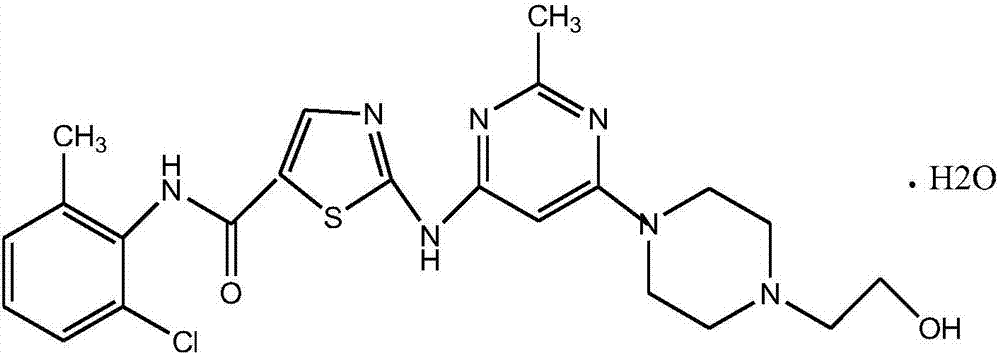
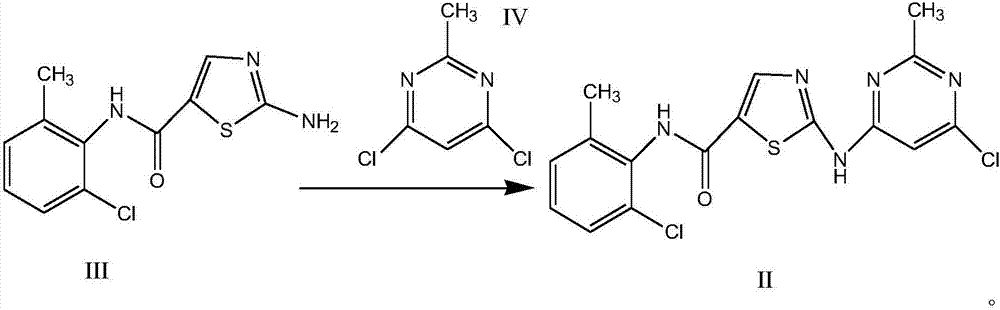
A kind of synthetic method of dasatinib intermediate
A technology for dasatinib and its intermediates, which is applied in the field of synthesis of N--2-[amino]-5-thiazolecarboxamide, the intermediate of dasatinib Product quality impact and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 8.73g of potassium hydride (30% content, 65.38mmol) to 80ml of tetrahydrofuran cooled to -25°C; after addition, stir for 10min; slowly add 5.00g of 2-amino-N-(2-chloro-6-methyl Phenyl)thiazole-5-carboxamide, add the process to control the temperature -25°C; add the tetrahydrofuran (7ml) solution containing 3.65g of 2-methyl-4,6-dichloropyrimidine prepared in advance to the reaction system at one time After stabilization, heat up, stir and react at -10°C for 4h; then slowly add 1mol / L hydrochloric acid dropwise to quench the reaction, adjust the pH to 6, control the temperature at 0-5°C, crystallize for 2h, centrifuge, wash with THF, and dry to obtain the crude product 7.27g. The yield is 98.7%, the purity is 99.95% (HPLC), and the maximum simple impurity content is 0.03%.
Embodiment 2
[0029] Add 2.84g of sodium hydride (60% content, 70.98mmol) to 70ml of tetrahydrofuran cooled to -30°C; after addition, stir for 10min; slowly add 5.00g of 2-amino-N-(2-chloro-6-methyl Phenyl)thiazole-5-carboxamide, add the process to control the temperature -30°C; add the tetrahydrofuran (10ml) solution containing 3.65g of 2-methyl-4,6-dichloropyrimidine prepared in advance to the reaction system at one time After stabilization, heat up, stir and react at -8°C for 6h; then slowly add 1mol / L hydrochloric acid dropwise to quench the reaction, adjust the pH to 6, control the temperature at 0-5°C, crystallize for 2h, centrifuge, wash with THF, and dry to obtain the crude product 7.15g. The yield is 97.1%, the purity is 99.90% (HPLC), and the maximum simple impurity content is 0.04%.
Embodiment 3
[0031]Add 2.19g of sodium amide to 70ml of tetrahydrofuran cooled to -20°C; after addition, stir for 10min; slowly add 5.00g of 2-amino-N-(2-chloro-6-methylphenyl)thiazole-5-methanol Amide, add process temperature control -20 ° C; add the tetrahydrofuran (10ml) solution containing 3.35g of 2-methyl-4,6-dichloropyrimidine prepared in advance to the reaction system at one time, and heat up after stabilization, -10 Stir the reaction at ℃ for 2 h; then slowly drop 1 mol / L hydrochloric acid to quench the reaction, adjust the pH to 5, control the temperature at 0-5 °C, crystallize for 3 h, centrifuge, wash with THF, and dry to obtain 7.13 g of crude product. The yield is 96.8%, the purity is 99.82% (HPLC), and the maximum simple impurity content is 0.06%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com