Polyethylene glycol-brefeldin A ester derivatives and preparation and applications of the derivatives
A technology of brefeldin and brefeldin, which is applied in the field of polyethylene glycol-brefeldin A ester derivatives, can solve the problems of poor water solubility, short plasma half-life, inability to apply anti-tumor therapeutic reagents, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
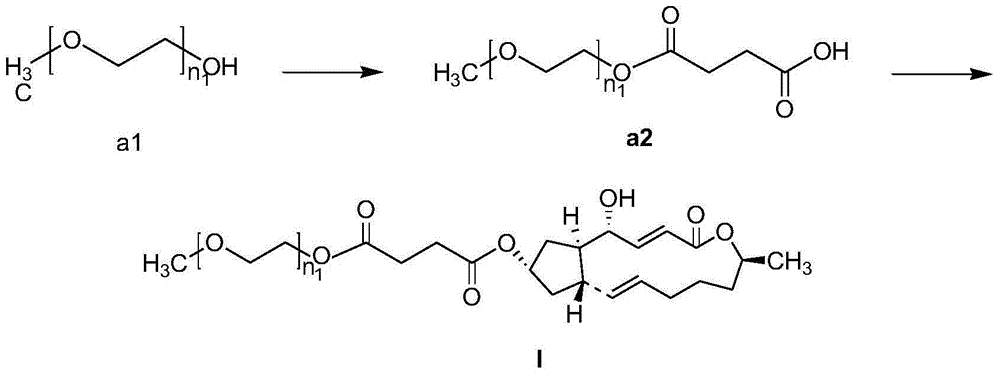
Embodiment 1
[0100] Embodiment 1: the preparation of (II-1)
[0101]
[0102] Under nitrogen protection, polyethylene glycol 1000 diacid ester (0.12g, 0.1mM) was added to a round-bottomed flask equipped with 25ml of anhydrous dichloromethane, stirred to completely dissolve, and brefeldin A (0.056g, 0.2 mM) was heated at 40°C until completely dissolved, EDC (0.058g, 0.3mM) and DMAP (0.012g, 0.1mM) were added in sequence, and the mixture was heated to reflux for reaction. The reaction process was monitored by TLC, and concentrated sulfuric acid was used for color development. After 24h, the reaction was terminated, and 30mlH was added to the reaction solution. 2 O, with CH 2 Cl 2 Extraction (3x15ml), the combined organic phase was washed with anhydrous Na 2 SO 4 Drying, filtration, the filtrate was concentrated to remove most of the solvent, separated by silica gel column chromatography, the eluent was a mixed solvent of dichloromethane: methanol (D / M) volume ratio=15:1, and the elue...
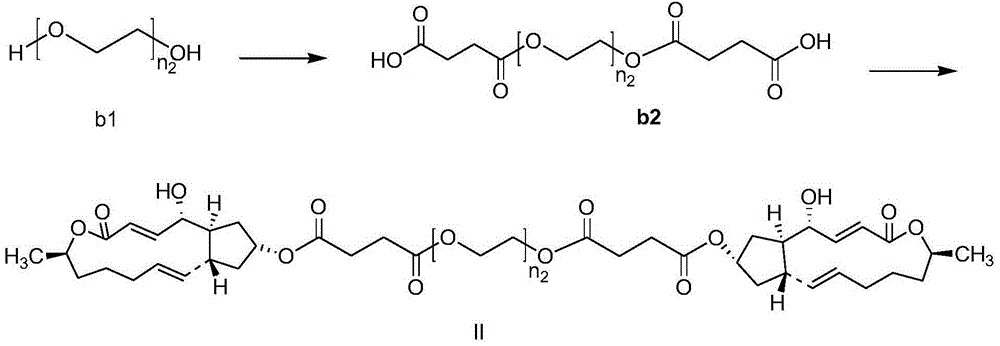
Embodiment 2
[0105] Embodiment 2: Preparation of 4b (II-2)
[0106]
[0107] Under the protection of nitrogen, polyethylene glycol 4000 diacid ester (0.41g, 0.1mM) was added to a round-bottomed flask equipped with 25ml of anhydrous dichloromethane, stirred to completely dissolve, and brefeldin A (0.056g, 0.2 mM) was heated at 40°C until completely dissolved, EDC (0.058g, 0.3mM) and DMAP (0.012g, 0.1mM) were added in sequence, mixed and heated to reflux for reaction. The reaction process was monitored by TLC, and concentrated sulfuric acid was used for color development. After 24h, the reaction was terminated, and 30mlH was added to the reaction solution. 2 O, with CH 2 Cl 2 Extraction (3x15ml), the combined organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, concentrate to remove most of the solvent, separate by silica gel column chromatography, the eluent is dichloromethane: methanol (D / M) volume ratio = 15:1 mixed solvent, collect the eluate containing the product, eva...
Embodiment 3
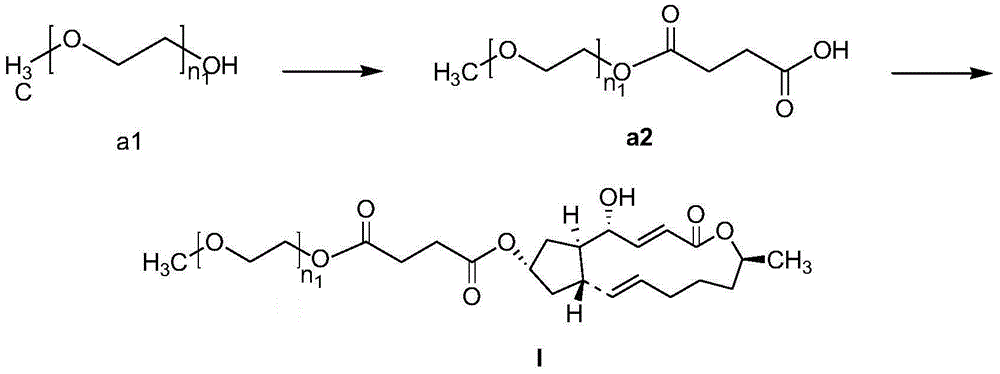
[0110] Embodiment 3: 4c (I-1) preparation
[0111]
[0112] Under nitrogen protection, monomethyl ether polyethylene glycol 1000-1-ester (0.11g, 0.1mM) was added to a round-bottomed flask containing 25ml of anhydrous dichloromethane, stirred to completely dissolve, and brefeldin A was added (0.056g, 0.2mM) was heated at 40°C until completely dissolved, EDC (0.058g, 0.3mM) and DMAP (0.012g, 0.1mM) were added in sequence, and the mixture was heated to reflux for reaction. The reaction process was monitored by TLC, and concentrated sulfuric acid was used for color development. After 24h, the reaction was terminated, and 30mlH was added to the reaction solution. 2 O, with CH 2 Cl 2 Extraction (3x15ml), the combined organic phase was washed with anhydrous Na 2 SO 4 Dry, filter, concentrate to remove most of the solvent, separate by silica gel column chromatography, the eluent is dichloromethane: methanol (D / M) volume ratio = 15:1 mixed solvent, collect the eluate containing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com