Bis carbonyl indole compound and synthesis method
A technology of biscarbonyl indole and a synthesis method, which is applied in the field of organic compound technology application, can solve problems such as application hindrance and toxicity, and achieve the effects of high reaction efficiency, mild reaction conditions and easy preparation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
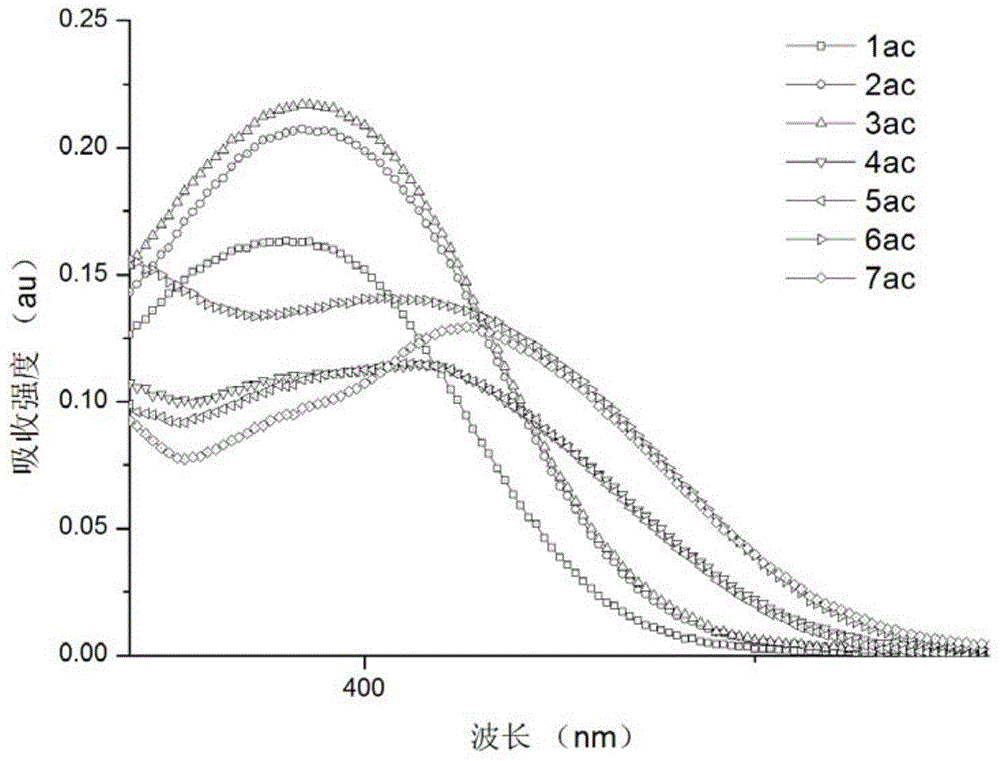
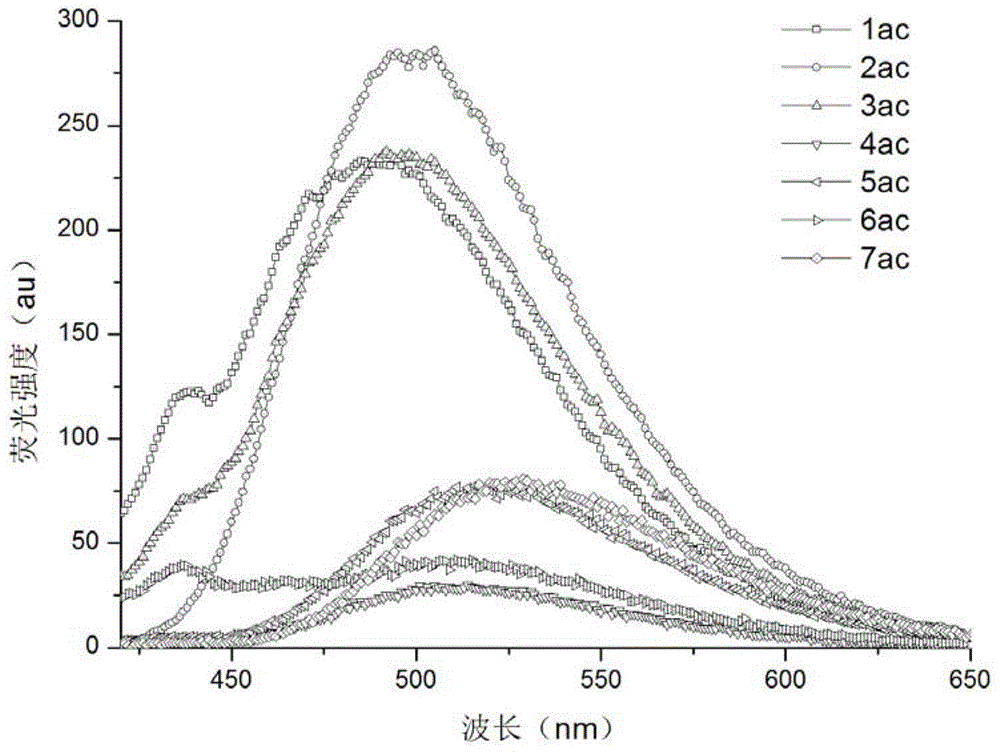
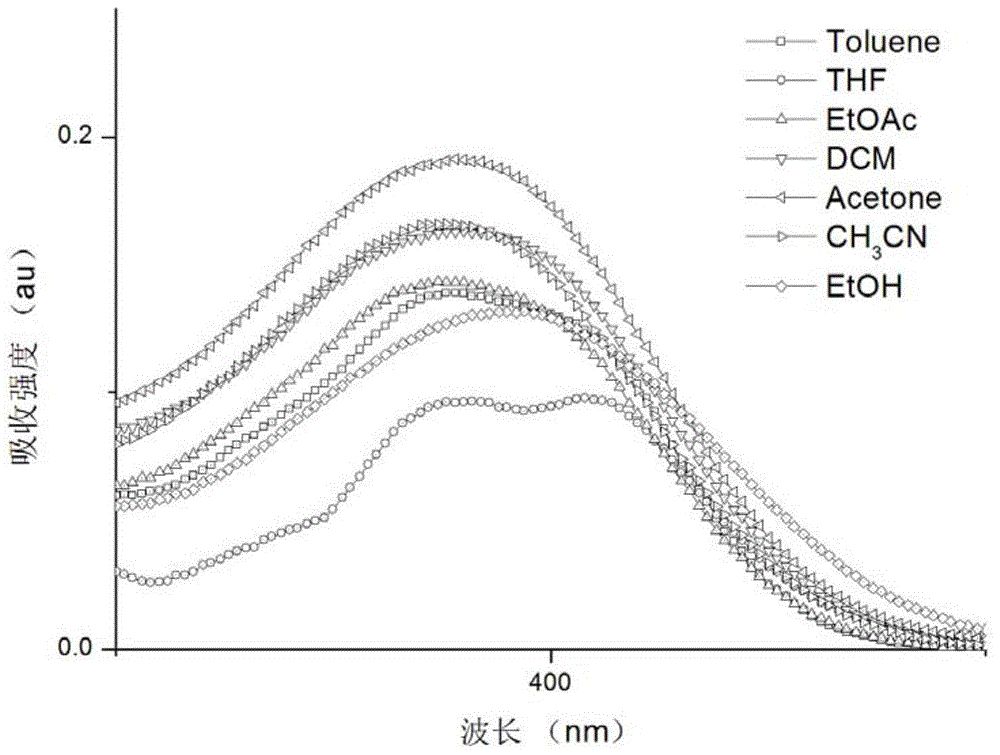
Image
Examples
Embodiment 1
[0043] Synthesis of Compound 1ab:
[0044]
[0045] The catalyst CuTC (0.02mmol, 10mol%) and α-hydroxyacetophenone 1a (0.2mmol, 1equiv., 27.2mg) and 1-methylindole 1b (0.6mmol, 78.7mg) were added to the reaction tube, and then added After the reaction solvent benzene (4 mL), the reaction was stirred at a reaction temperature of 80° C. for 6 hours in an oxygen atmosphere. After monitoring the reaction by thin-layer chromatography, the product 1ab (43.4mg) yield was obtained after direct column chromatography separation: 83%;1 H NMR (400MHz, CDCl 3 )δ8.53–8.42(m,1H),8.10(dd,J=5.1,3.4Hz,2H),7.80(s,1H),7.67–7.58(m,1H),7.49(t,J=7.7Hz ,2H),7.43–7.36(m,3H),3.83(s,3H); 13 C NMR (100MHz, CDCl 3 )δ193.7, 187.5, 139.5, 137.7, 134.2, 133.5, 130.3, 128.7, 126.4, 124.2, 123.5, 122.7, 112.9, 109.9, 33.7. MS (EI) m / z 263 (M + ).
Embodiment 2
[0047] Synthesis of compound 2ab:
[0048]
[0049] The catalyst CuTC (0.02mmol, 10mol%) and α-hydroxyacetophenone 1a (0.2mmol, 1equiv., 27.2mg) and 1-allylindole 2b (0.6mmol, 94.8mg) were added in the reaction tube, and then After adding the reaction solvent benzene (4 mL), the reaction was stirred at a reaction temperature of 80° C. for 6 hours in an oxygen atmosphere. After the end of the reaction was monitored by thin-layer chromatography, product 2ab (39.6 mg) was obtained after direct column chromatography separation. Yield: 69%; 1 H NMR (400MHz, CDCl 3 )δ8.55–8.45(m,1H),8.16–8.07(m,2H),7.85(s,1H),7.67–7.59(m,1H),7.49(dd,J=10.6,4.8Hz,2H) ,7.42–7.33(m,3H),6.05–5.91(m,1H),5.25(ddd,J=17.8,13.7,0.8Hz,2H),4.75(dt,J=5.6,1.4Hz,2H); 13 C NMR (100MHz, CDCl 3 )δ193.6, 187.6, 138.5, 137.1, 134.2, 133.4, 131.4, 130.3, 128.7, 126.5, 124.2, 123.5, 122.7, 119.3, 113.1, 110.4, 49.7. HRMS (EI) Calcd for C 19 h 15 NO 2 289.1103,Found 289.1102.
Embodiment 3
[0051] Synthesis of compound 3ab:
[0052]
[0053] The catalyst CuTC (0.02mmol, 10mol%) and α-hydroxyacetophenone 1a (0.2mmol, 1equiv., 27.2mg) and 1-benzyl indole 3b (0.6mmol, 124.2mg) were added to the reaction tube, and then added After the reaction solvent benzene (4 mL), the reaction was stirred at a reaction temperature of 80° C. for 6 hours in an oxygen atmosphere. After the end of the reaction was monitored by thin-layer chromatography, product 3ab (47.8 mg) yield was obtained after direct column chromatography separation: 71%; 1 H NMR (400MHz, CDCl 3 )δ8.51(d,J=7.8Hz,1H),8.18–8.06(m,2H),7.92(s,1H),7.67–7.59(m,1H),7.50(t,J=7.7Hz,2H ),7.38(m,1H),7.36–7.27(m,5H),7.20–7.13(m,2H),5.33(s,2H); 13 C NMR (100MHz, CDCl 3 )δ193.5,187.6,138.8,137.2,135.1,134.2,133.4,130.3,129.1,128.7,128.3,127.0,126.6,124.3,123.5,122.8,113.3,110.6,51.1. 23 h 17 NO 2 339.1259, Found 339.1262.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com