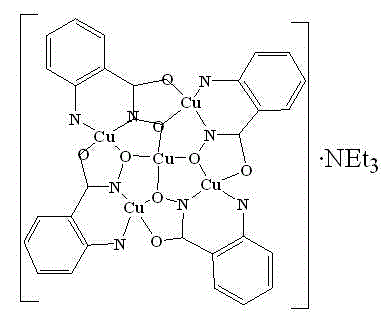
O-amino benzohydroxamic acid copper compound, preparation method and applications thereof
A technology of copper anthranilate hydroxamate and copper anthranilate hydroxamate is applied in the fields of copper anthranilate hydroxamate compound, preparation and application, and achieves high anticancer activity, simple preparation method and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 0.2mmol anthranilic acid hydroxamic acid (H 2 anha) and 0.2 mmol Cu(OAc) 2 ·H 2 O mixed and dissolved in 20 mL CH 3 OH, stirred at room temperature for 15 minutes, then added 0.4 mmol of triethylamine, and continued to stir at room temperature for 5-6 hours. A dark green solution was obtained by filtration, and the filtrate evaporated naturally. Dark green crystals were obtained after three weeks. The melting point is greater than 300°C. The highest yield of this compound was 68.6%.
[0015] The copper compound of the present invention is analyzed by X-single crystal diffraction, and the obtained crystallographic data are as follows: the compound belongs to the monoclinic crystal system, and the space group is P 2(1) / c , the cell parameters are: a = 9.5607(8) ?, b = 18.9638(17) ?, c = 11.8070(11) ?, α = 90°, β =107.241(2)°, gamma = 90°, V = 2044.5 ? 3 , Z = 8, D c = 1.647 Mg m -3 , mu =2.113mm -1 , F(000) = 1031, 2.81 1 = 0.0429, w R 2 ...
Embodiment 2
[0017] 0.2mmol anthranilic acid hydroxamic acid (H 2 anha) and 0.2mmol Cu(OAc) 2 ·H 2 O mixed and dissolved in 20mL CH 3 OH, stirred at room temperature for 15 minutes, then added 0.4 mmol of triethylamine, and continued to stir at room temperature for 10 hours. A dark green solution was obtained by filtration, and the filtrate evaporated naturally. Dark green crystals were obtained after three weeks. The melting point is greater than 300°C. The highest yield of the compound was 67.2%.
Embodiment 3
[0019] 0.2mmol anthranilic acid hydroxamic acid (H 2 anha) and 0.2mmol Cu(OAc) 2 ·H 2 O mixed and dissolved in 20mL CH 3 OH, stirred at room temperature for 15 minutes, then added 0.4 mmol of triethylamine, and continued to stir at room temperature for 14 hours. A dark green solution was obtained by filtration, and the filtrate evaporated naturally. Dark green crystals were obtained after three weeks. The melting point is greater than 300°C. The highest yield of the compound was 66.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com