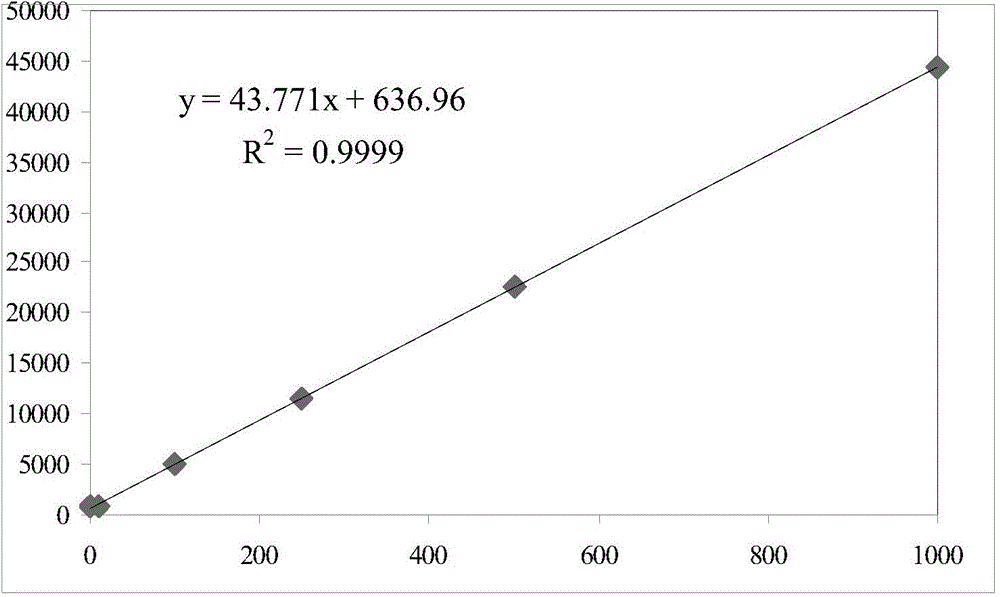
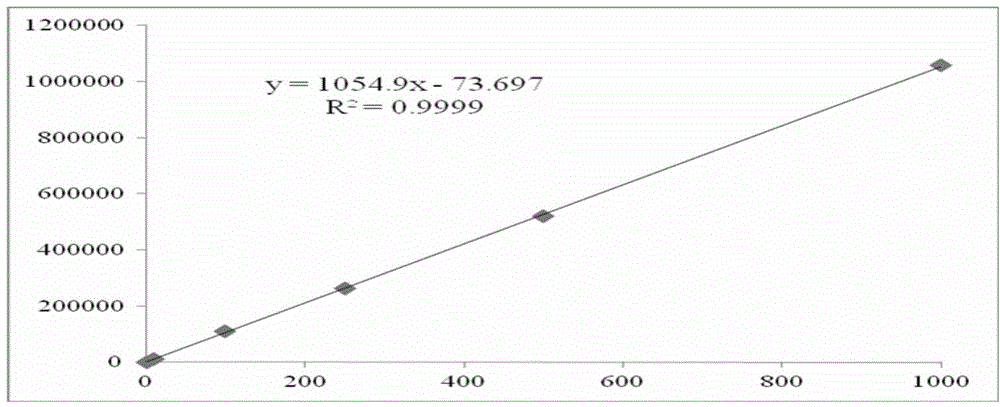
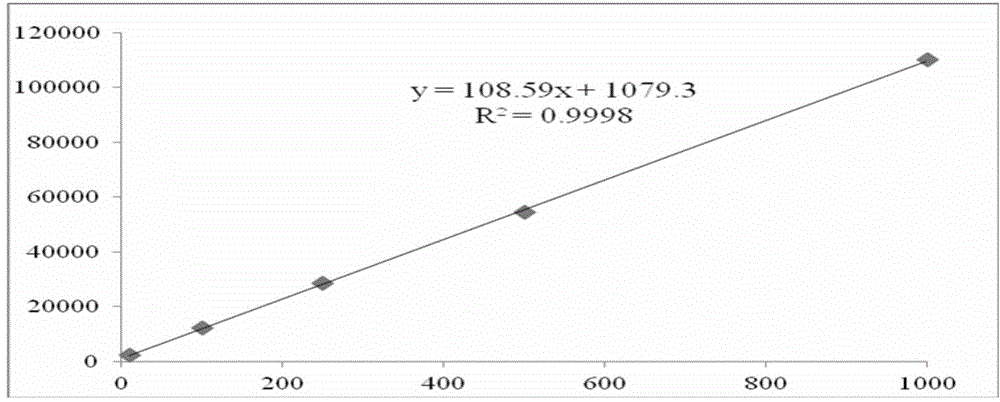
Method for analyzing cathinone, methcathinone and 4-methylmethcathinone in biological sample by liquid chromatography-mass spectrometry
A technology of methcathinone and biological samples, which is applied in the field of drug detection in the field of criminal investigation, can solve the problems of complex biological sample system, troublesome detection work, and many interferences, and achieve high-efficiency qualitative and quantitative, good purification effect, and high detection accuracy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Liquid-mass spectrometry analyzes the method for cathinone, methcathinone, 4-methyl methcathinone in urine, it comprises the steps:
[0049] (1) Preparation of sample solution;
[0050] (2) Preparation of standard working solution;
[0051] (3) Setting of detection conditions;
[0052] (4) Liquid-mass spectrometry determination;
[0053] (5) Calculation of experimental results, where,
[0054]Step (1): Take three blank urine samples of healthy volunteers, add cathinone, methcathinone and 4-methyl methcathinone respectively to prepare urine sample 1 (cathinone concentration is 500ng / mL ), urine sample two (concentration of methcathinone is 500ng / mL) and urine sample three (concentration of 4-methyl methcathinone is 500ng / mL), respectively, according to the volume ratio of sample and water of 2:1 After proportional dilution, add protein precipitating agent acetonitrile, protein precipitating agent is added until no precipitation is formed in the sample, then after ult...
Embodiment 2
[0075] Liquid-mass spectrometry analyzes the method for cathinone, methcathinone, 4-methyl methcathinone in blood, and it comprises the steps:
[0076] (1) Preparation of sample solution;
[0077] (2) Preparation of standard working solution;
[0078] (3) Setting of detection conditions;
[0079] (4) Liquid-mass spectrometry determination;
[0080] (5) Calculation of experimental results, where,
[0081] Step (1): Take three blank blood samples from healthy volunteers, add cathinone, methcathinone and 4-methyl methcathinone respectively to prepare urine sample 1 (cathinone concentration is 500ng / mL) , Urine sample 2 (the concentration of methcathinone is 500ng / mL) and urine sample 3 (the concentration of 4-methyl methcathinone is 500ng / mL), after dilution with water at a volume ratio of 1:1, Add the protein precipitant acetonitrile, the protein precipitant is added until no precipitation is formed in the sample, then ultrasonically oscillate for 15 minutes, then centrifuge...
Embodiment 3
[0088] The method for analyzing cathinone, methcathinone, 4-methylmethcathinone and common adulterants thereof in blood by liquid chromatography-mass spectrometry comprises the following steps:
[0089] (1) Preparation of sample solution;
[0090] (2) Preparation of standard working solution;
[0091] (3) Setting of detection conditions;
[0092] (4) Liquid-mass spectrometry determination;
[0093] (5) Calculation of experimental results, where,
[0094] The common adulterants are norephedrine, amphetamine, amphetamine-d8, methamphetamine, methamphetamine-d8, nicotine, ephedrine, MDMA, MDMA, coffee Ketamine, Demerol, Tramadol, Methorphan, Nitrozepam, Valium, Morphine, Estazolam, Codeine, Cocaine, Alprazolam, Methadone, Cannabidiol, Thebaine, THC phenol, cannabidiol, monoacetylmorphine, fentanyl, papaverine, acetylcodeine, triazolam, heroin, narcotine.
[0095] Step (1): Take blank blood from healthy volunteers, add methcathinone to prepare blood sample 1 (methcathinone co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com