Freeze drying method of omeprazole sodium for injection and omeprazole sodium for injection
A technology of omeprazole sodium and drying method, which is applied in the direction of freeze-drying transportation and powder transportation, etc., which can solve the problems of unguaranteed product quality, unfavorable preservation, instability, etc., and achieve qualified quality, favorable preservation, and roundness Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] The specific technical scheme and experimental steps are as follows:
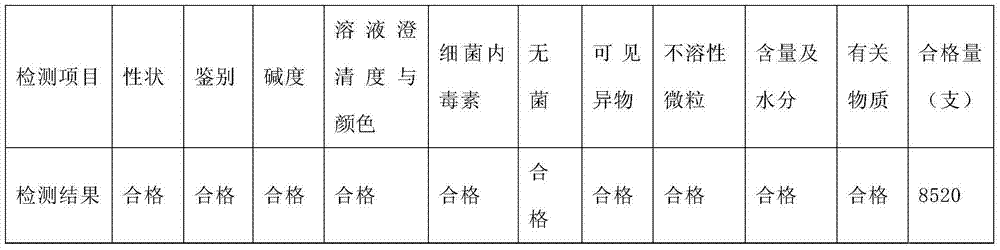
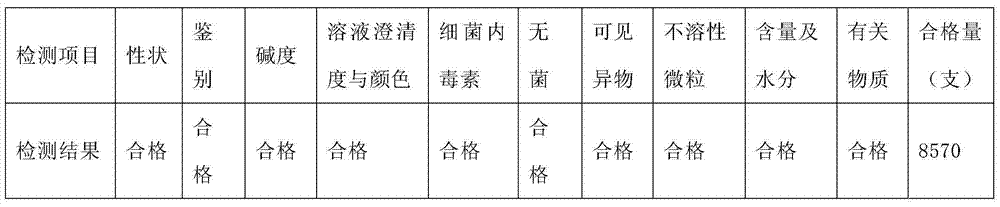
[0014] 1. The first trial
[0015] 1.1 Test time: April 5 to April 7, 2012
[0016] 1.2 Product batch number: 20120401
[0017] 1.3 Name of product batch number: Omeprazole Sodium for Injection
[0018] 1.4 Product specification: 40mg
[0019] 1.5 Test steps:
[0020] 1.5.1 Put the prepared and filtered medicinal liquid through the filling half-pressed stopper, put the half-pressed product 9500 bottles into the freeze dryer, and close the freeze dryer door.
[0021] 1.5.2 Set the parameters of the freeze dryer:
[0022] 1.5.2.1 Pre-freezing parameter setting: it takes 1.0 hours for the product to cool down from normal temperature to -40°C, and it is kept at this temperature for 1.0 hours;
[0023] 1.5.2.2 Sublimation drying parameter setting: the temperature of the product from -40℃ to -20℃, the required time is 10.0 hours, and the temperature is kept for 5.0 hours;
[0024] 1.5.2.3 Analysis o...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap