Application of blood vessel inhibiting polypeptides with integrin affinity and bonding capability and MMPs (matrix metalloproteinases) inhibiting capability
A technology of protease inhibition and binding ability, applied in the field of medicine, can solve the problems of poor drug targeting and poor therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
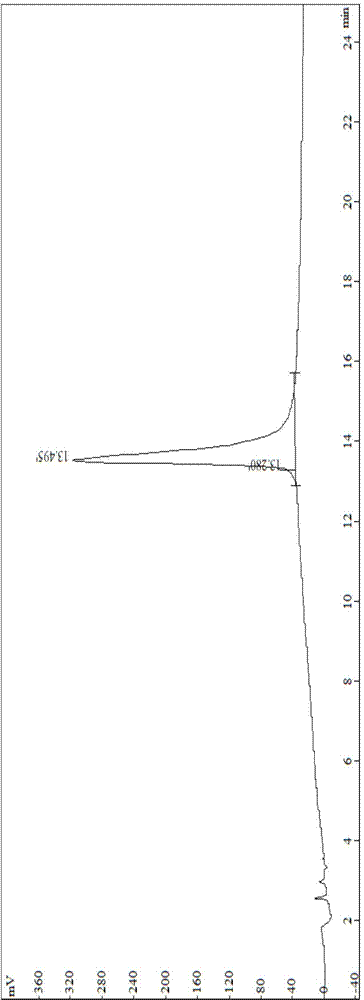
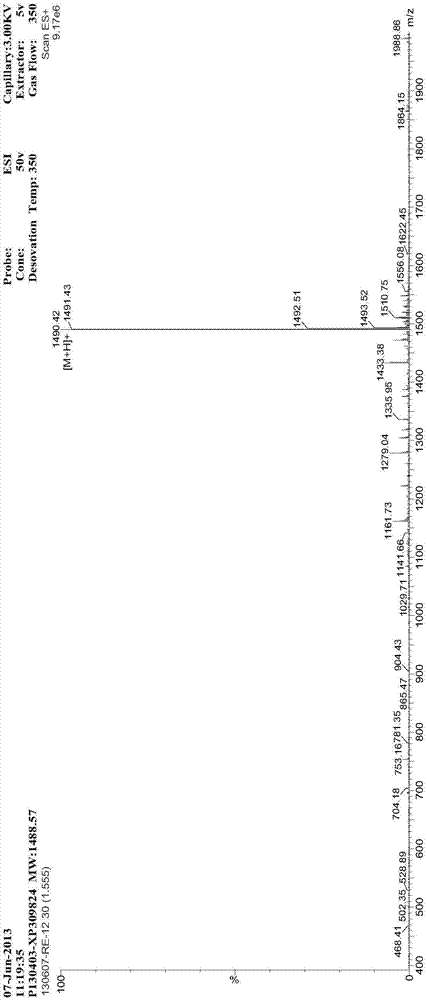
[0088] Preparation and Test of Synthetic Peptide I of Vascular Inhibitory Polypeptide
[0089] The solid-phase synthesis method of polypeptide I Arg-Gly-Asp-Gly-Gly-Gly-Gly-Pro(D-Pyr)-(D-Cys)--Bip-Arg-Gly-Glu, which starts with wang resin As raw materials, one peptide to fourteen peptides are sequentially connected with protected amino acids. After the peptide connection is completed, the peptide is fully washed, and then the peptide is cut and post-processed to obtain the crude product of polypeptide I. The crude product was purified twice with preparative HPLC, and finally concentrated and freeze-dried to obtain the pure product. The purity of the polypeptide was determined by analytical RP-HPLC. The method can not only ensure the synthesis efficiency, but also improve the product purity.
[0090] Specific steps are as follows:
[0091] 1. Synthesis:
[0092] Weigh 13g of Wang resin (Wang resin), pour it into a 1L glass sand core reaction column, add CH 2 Cl 2 130mL to...
Embodiment 2
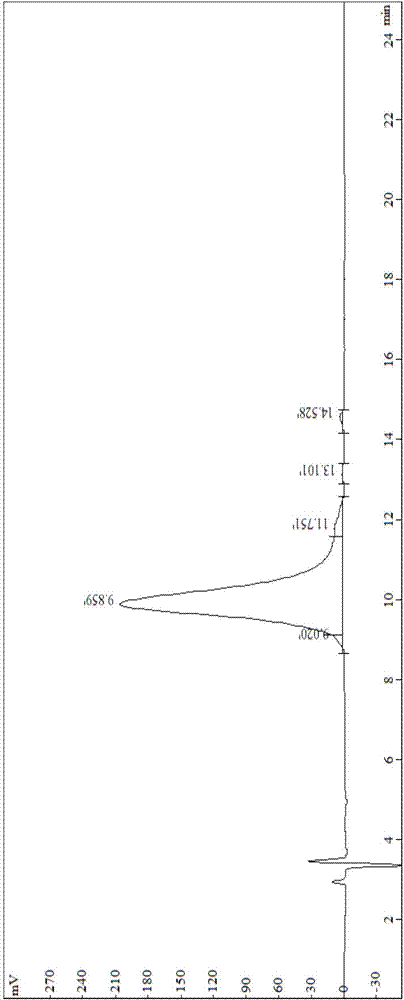
[0123] Preparation and Testing of Synthetic Peptide II of Vasoinhibitory Polypeptide
[0124] The solid-phase synthesis method of Pro-(D-Pyr)-(D-Cys)-Bip-Arg-Gly-Glu-Gly-Gly-Gly-Gly-Gly-Arg-Gly-Asp, which uses Fmoc-Asp(OtBu)- Wang resin was used as the starting material, and the dipeptide to tetradecapeptide were inoculated sequentially with protected amino acids. After the peptide incorporation was completed, it was fully washed, and then the peptide was cut and post-treated to obtain the crude polypeptide II. The crude product was purified twice with preparative HPLC, and finally concentrated and freeze-dried to obtain the pure product. Specific steps are as follows:
[0125] 1. Synthesis:
[0126] Weigh 14.5g of Fmoc-Asp(OtBu)-wang resin (Wang resin prepacked resin), pour it into a 1L glass sand core reaction column, add CH 2 Cl 2 145mL to fully expand the resin.
[0127] Uncapping: Add 25mL of capping solution, seal it and place it in a shaker for 5min to react, the t...
Embodiment 3
[0156] Vaso-inhibitory polypeptides in vivo immune protection in adjuvanted rat arthritis animal model
[0157]An animal model of adjuvant arthritis in rats was constructed to study the therapeutic effect of angioinhibitory polypeptide on adjuvant arthritis (Adjuvant arthritis, AA) rats. Rats were used as test animals, and 63 clean-grade wistar rats (provided by the Comparative Medicine Center of Yangzhou University, animal production license number: SCXK (Su) 2012-0004), male, with a body weight of 150g-180g, were randomly divided into 9 groups, respectively blank control group, model control group, 3 low, medium and high dose groups of polypeptide I (3, 6, 9 mg / kg), 3 low, medium and high dose groups of polypeptide II (3, 6, 9 mg / kg) and positive Drug control group (methotrexate 1mg / kg). Except for the blank group, rat AA models were established in each experimental group on the 0th day by injecting complete Freund's adjuvant containing inactivated Mycobacterium tuberculosi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com