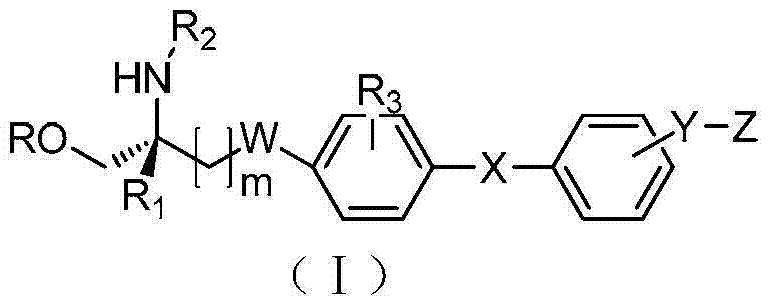
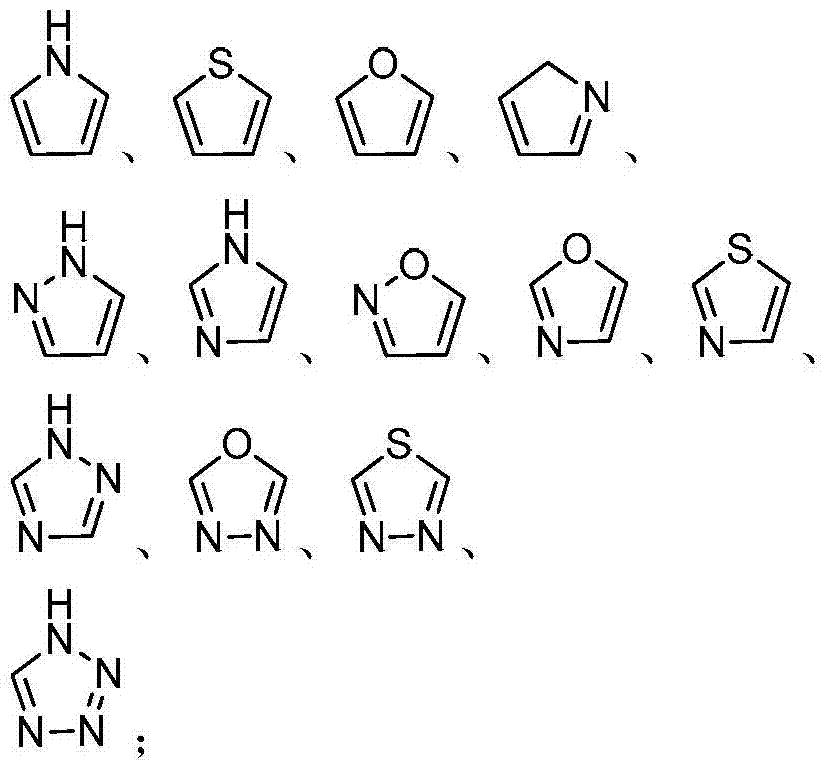
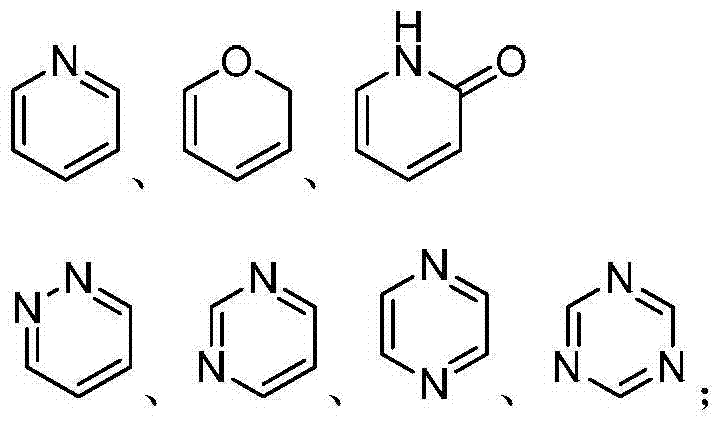
Amino propanediol derivatives, preparation method, drug compositions and uses thereof
A compound, alkyl technology, applied in the field of medicine, can solve problems such as adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] This experiment demonstrates the preparation of 2-amino-2-((4-((4-benzyloxyphenyl)thio)phenoxy)methylene)-1,3-propanediol hydrochloride
[0107]
[0108] (1-1) Preparation of 4-((4-benzyloxy)phenylthio)phenol
[0109]
[0110] Put 4,4-diphenylsulfide diphenol (10g, 45.8mmol, 1equiv) into a 250mL three-necked flask with a stirrer. The three-necked flask is equipped with a spherical condenser and a constant-pressure dropping funnel. Put 100mL N,N-dimethylformamide (DMF) into the reactor, stir until dissolved, put potassium hydroxide (2.827g, 50.4mmol, 1.1equiv), potassium iodide (380mg, 2.29mmol, 0.05equiv) into the reactor in turn, Stir for 5 minutes, add a DMF solution of benzyl bromide (6.0 mL benzyl bromide + 20 mL DMF) dropwise into the reactor, and the dropwise addition is completed in 4 hours, continue the reaction for 8 hours, and raise the temperature to 40°C for 4 hours. After the reaction was completed, the reaction liquid was poured into water, stirred ...
Embodiment 2
[0121] This experiment demonstrates that 2-amino-2-(4-(4-(4-((4-methyl)phenyl)phenylthio)phenoxymethylene))-1,3-propanediol hydrochloride preparation
[0122]
[0123] (2-1) Preparation of 4-((4-hydroxy)phenylthio)phenyltrifluoromethanesulfonate
[0124]
[0125]Put 4,4,-diphenyl sulfide diphenol (2.00g) and acetone (35mL) into a 100mL eggplant-shaped bottle in turn, cool to -15°C in an ice-salt bath, and add trifluoromethanesulfonic anhydride dropwise to the reaction solution The dichloromethane solution (1.6mL trifluoromethanesulfonic anhydride + 2mL dichloromethane), was added dropwise in 20 minutes, and reacted at room temperature for 3 hours. After the reaction, the reaction solution was poured into ice water, extracted with dichloromethane (20 mL×3), dried over anhydrous sodium sulfate, and the solvent was distilled off. Flash column chromatography gave 1.034 g off-white solid with a yield of 32.2%. 1 HNMR (400MHz, Acetone-d 6 )δ(ppm):8.890(s,1H),7.44(d,J=8.4,2...
Embodiment 3
[0136] This experiment demonstrates that 2-amino-2-(4-(4-((2-n-propyl)oxazol-4-yl)phenylthio)phenoxymethylene)-1,3-propanediol hydrochloride preparation of
[0137]
[0138] (3-1) 4-(Bromoacetyl) bromobenzene
[0139]
[0140] Bromobenzene (13.0mL, 0.127mmol, 1equiv), dichloromethane (200mL), and bromoacetyl bromide (11.0mL, 0.127mmol, 1equiv) were successively put into a 500mL three-necked flask, cooled to 0°C in an ice bath, and batch Aluminum trichloride powder (34.0 g, 0.254 mmol, 2 equiv) was added once, and the addition was completed in 1 hour, and the reaction was carried out at room temperature for 2 hours. After the reaction is complete, pour the reaction solution into 2N hydrochloric acid with ice cubes, stir at room temperature for 1 hour, separate the liquids, extract with dichloromethane (50mL×2), dry over anhydrous sodium sulfate, and distill off the dichloromethane to obtain 26.44g of yellow Solid, yield 74.9%. 1 H NMR (300MHz, CDCl 3 )δ (ppm): 7.86 (d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com