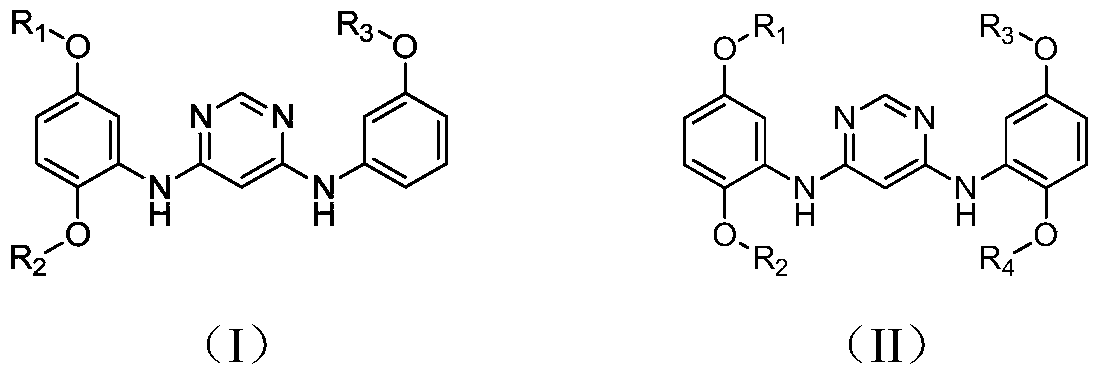
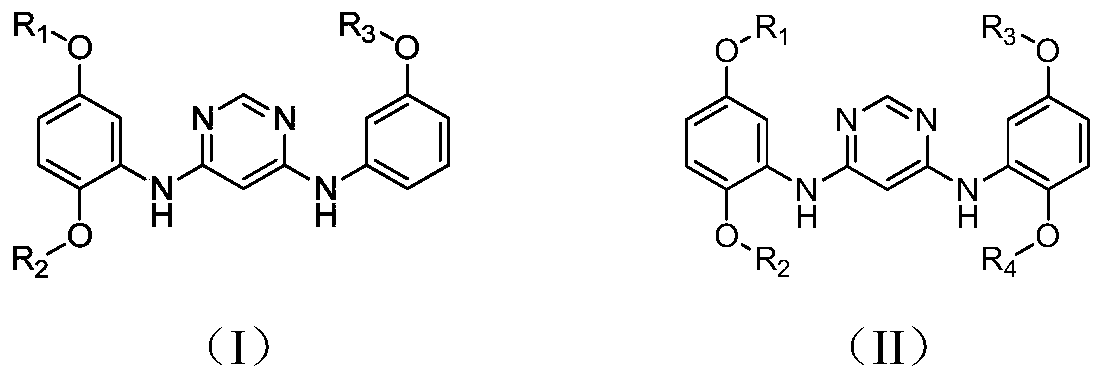
A kind of 4,6-pyrimidinediamine compound and its preparation method and application
A pyrimidinediamine and compound technology, which is applied in the field of 4,6-pyrimidinediamine compounds and their preparation, can solve the problems of unclear structure-activity relationship of EGFR inhibitors, inability to effectively inhibit EGFR kinase, and different effects, etc. Achieving excellent inhibitory effect, beneficial to industrial production, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
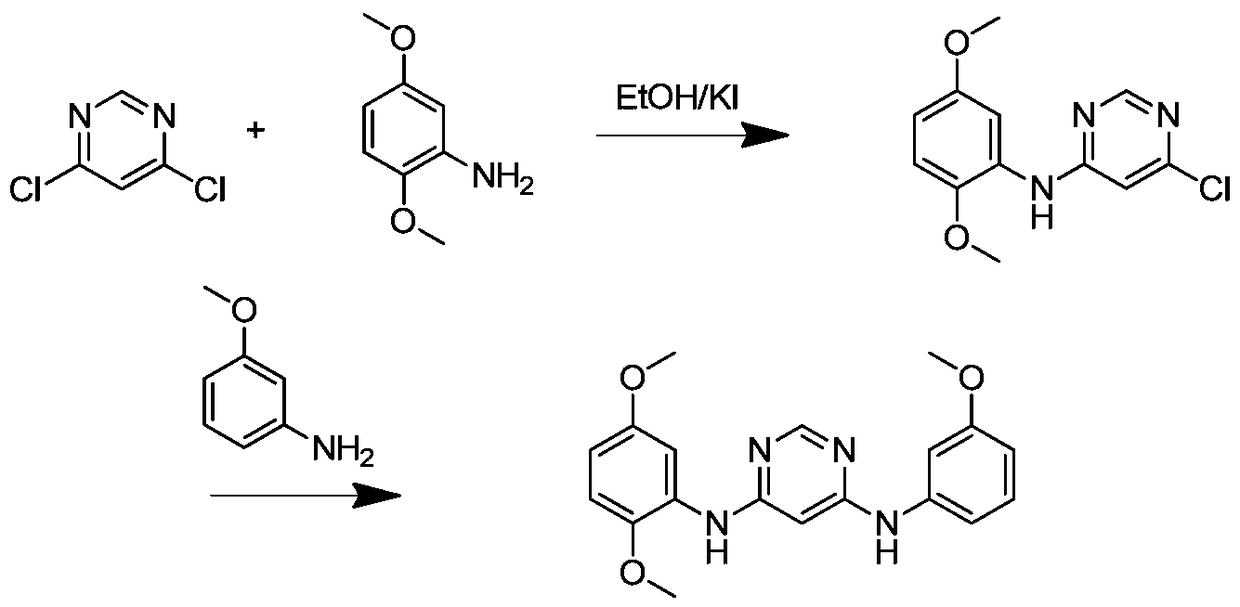
[0034] Example 1 prepares N 4 -(2,5-Dimethoxyphenyl)-N 6 -(3-Methoxyphenyl)-pyrimidinediamine
[0035]
[0036] Take 1g of 4,6-dichloropyrimidine and put it into a reaction bottle, add 30mL of ethanol, appropriate amount of KI, dissolve it by ultrasonic, stir with magnetic force, heat to reflux at 80°C, take 1.275g of 2,5-dimethoxyaniline and put it in another circle Bottom flask, and add 10mL ethanol to dissolve. Add 5 drops every 5 minutes to the 4,6-dichloropyrimidine solution, continue to reflux for 1-2 hours after the drop, filter with suction, and recrystallize to obtain 6-chloro-N-(2,5-dimethoxybenzene base)-pyrimidin-4-amine. Calculated yield 78-82%.
[0037] Put 100 mg of 6-chloro-N-(2,5-dimethoxyphenyl)-pyrimidin-4-amine into a flask, add 120 mg of 3-methoxyaniline and 30 mg of p-toluenesulfonic acid to dissolve in In 30 mL of ethanol, stir, heat and reflux at 80°C for 6 hours, take the reaction solution for TLC running, and observe the completion of the reac...
Embodiment 2
[0039] Embodiment 2 prepares N 4 , N 6 -(2,5-Dimethoxyphenyl)-pyrimidinediamine
[0040]
[0041] Take 1g of 4,6-dichloropyrimidine and put it into a reaction bottle, add 30mL of ethanol and 0.5mol p-toluenesulfonic acid, dissolve it by ultrasonic, put it into a magnet, stir, heat and reflux at 80°C, and take 2mol of 2,5-dimethyl The oxyaniline was dissolved, stirred by magnetic force, and heated to reflux at 80°C for 6 hours. The reaction solution was taken for TLC running, and the complete reaction was observed under ultraviolet fluorescence. Suction filtration, recrystallization to get N 4 , N 6 -(2,5-Dimethoxyphenyl)-pyrimidinediamine crude. Add saturated NaHCO 3 , stirred for 1 hour, filtered with suction, and passed through a chromatographic column to obtain a refined product. The calculated yield was 75%-82%.
[0042] Melting point: 187°C. NMR data (d 6 -DMSO):3.669(s,6H,5'-Ph-OCH 3 ×2),3.752(s,6H,2'-Ph-OCH 3 ×2),6.257(s,1H,-NH),6.551-6.597(m,2H,5'-PhH),6...
Embodiment 3
[0043] Embodiment 3 The compounds of the present invention are tested for the inhibitory activity of different EGFR kinases
[0044] The method used in the experiment is Caliper Mobility Shift Assay, which is a detection platform based on the migration detection technology of microfluidic chip technology. Experimental steps: configure 1x kinase reaction buffer (50mM HEPES, pH7.5; 0.0015% Brij-35; 10mM MgCl 2 , 10mM MnCl 2 ; 2Mm DTT) and kinase reaction stop solution (100mM HEPES, pH7.5; 0.015% Brij-35; 0.2% Coating Reagent#3; 50mM EDTA); 10 times) add 10 μl of 2.5x substrate peptide solution (add kinase in 1x kinase reaction buffer), incubate at room temperature for 10 min, then add 10 μl of 2.5x substrate peptide solution (add FAM-labeled peptide in 1x kinase reaction buffer and ATP), add 25 μl kinase reaction stop solution after reacting at 28°C for a specific time. Test and collect data on Caliper, inhibition rate of kinase activity=[(max-conversion) / (max-min)]×100. "ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com