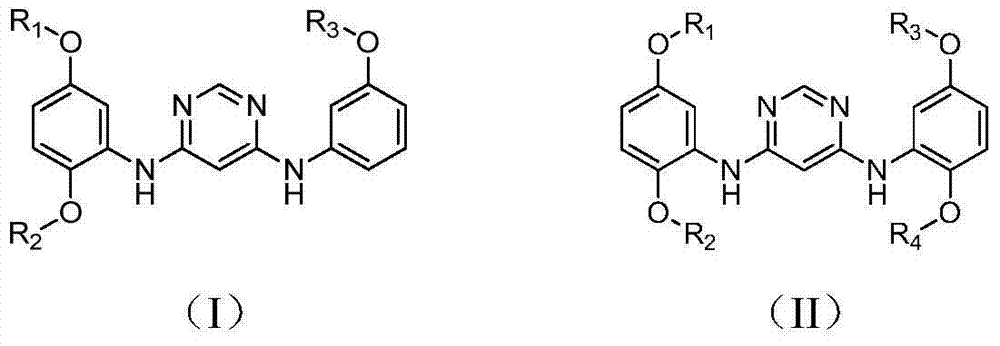
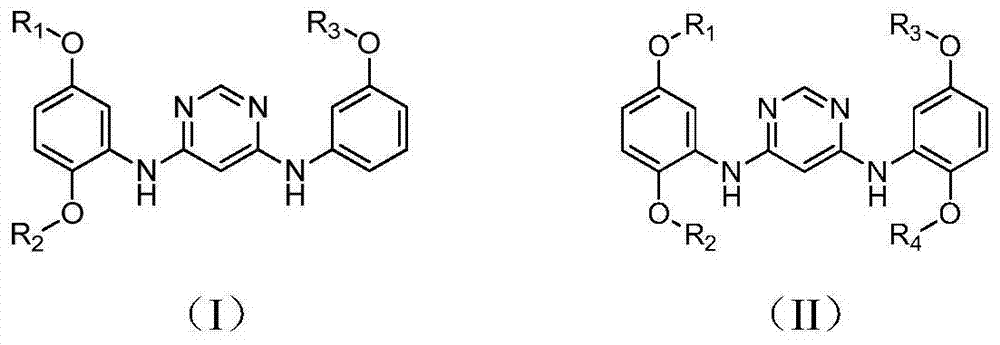
4,6-pyrimidine diamine compound and preparing method and application thereof
A pyrimidinediamine and compound technology, applied in the field of 4,6-pyrimidinediamine compounds and their preparation, can solve the problems of unclear structure-activity relationship of EGFR inhibitors, different effects, inability to effectively inhibit EGFR kinase, and the like, To achieve the effect of excellent inhibitory effect, good inhibitory activity and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
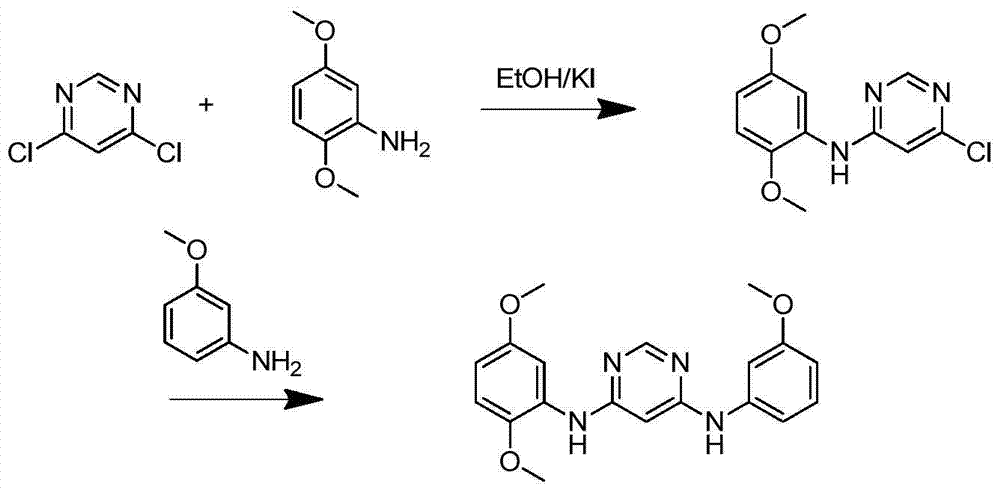
[0034] Example 1 Preparation of N 4 -(2,5-Dimethoxyphenyl)-N 6 -(3-Methoxyphenyl)-pyrimidinediamine
[0035]
[0036] Take 1g of 4,6-dichloropyrimidine into the reaction flask and add 30mL of ethanol, an appropriate amount of KI, dissolve by ultrasonic, magnetic stirring, heat to reflux at 80°C, take 1.275g of 2,5-dimethoxyaniline and place in another circle Add 10mL ethanol to the bottom flask to dissolve. Add 5 drops every 5 minutes to the 4,6-dichloropyrimidine solution, continue to reflux for 1-2 hours after dropping, filter with suction and recrystallize to obtain 6-chloro-N-(2,5-dimethoxybenzene) Yl)-pyrimidin-4-amine. The calculated yield is 78-82%.
[0037] Take 100mg of 6-chloro-N-(2,5-dimethoxyphenyl)-pyrimidin-4-amine into the flask, and add 120mg of 3-methoxyaniline and 30mg of p-toluenesulfonic acid to dissolve In 30 mL of ethanol, stir and heat to reflux at 80° C. for 6 hours, take the reaction solution for TLC run, and observe that the reaction is complete under u...
Embodiment 2
[0039] Example 2 Preparation of N 4 , N 6 -(2,5-Dimethoxyphenyl)-pyrimidinediamine
[0040]
[0041] Take 1g of 4,6-dichloropyrimidine into the reaction flask and add 30mL ethanol, 0.5mol p-toluenesulfonic acid, dissolve it ultrasonically, put it in a magnet, stir and heat to reflux at 80℃, take 2mol 2,5-dimethyl The oxyaniline was dissolved, stirred magnetically, and heated to reflux at 80°C for 6 hours. Take the reaction solution and run the TLC plate, and observe that the reaction is complete under ultraviolet fluorescence. Suction filtration, recrystallization to get N 4 , N 6 -(2,5-Dimethoxyphenyl)-pyrimidinediamine crude product. Add saturated NaHCO 3 , Stir for 1 hour, filter with suction, and pass through a chromatography column to obtain a refined product. The calculated yield is 75%-82%.
[0042] Melting point: 187°C. NMR data (d 6 -DMSO): 3.669(s,6H,5’-Ph-OCH 3 ×2),3.752(s,6H,2’-Ph-OCH 3 ×2), 6.257 (s, 1H, -NH), 6.551-6.597 (m, 2H, 5'-PhH), 6.931-6.946 (m, 2H, 3'-PhH...
Embodiment 3
[0043] Example 3 Test of the inhibitory activity of the compounds of the present invention on different EGFR kinases
[0044] The method used in the experiment is Caliper Mobility Shift Assay, which is a detection platform based on the migration detection technology of microfluidic chip technology. Experimental steps: configure 1x kinase reaction buffer (50mM HEPES, pH7.5; 0.0015% Brij-35; 10mM MgCl 2 , 10mM MnCl 2 2Mm DTT) and kinase reaction termination solution (100mM HEPES, pH7.5; 0.015% Brij-35; 0.2% Coating Reagent#3; 50mM EDTA); in 5μl of 5x concentration compound solution (use DMSO solution, diluted with water 10 times) add 10μl of 2.5x substrate peptide solution (add kinase in 1x kinase reaction buffer), incubate at room temperature for 10 minutes, then add 10μl of 2.5x substrate peptide solution (add FAM labeled peptide in 1x kinase reaction buffer And ATP), add 25μl of kinase stop solution after reacting at 28°C for a specific time. Test and collect data on Caliper, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com