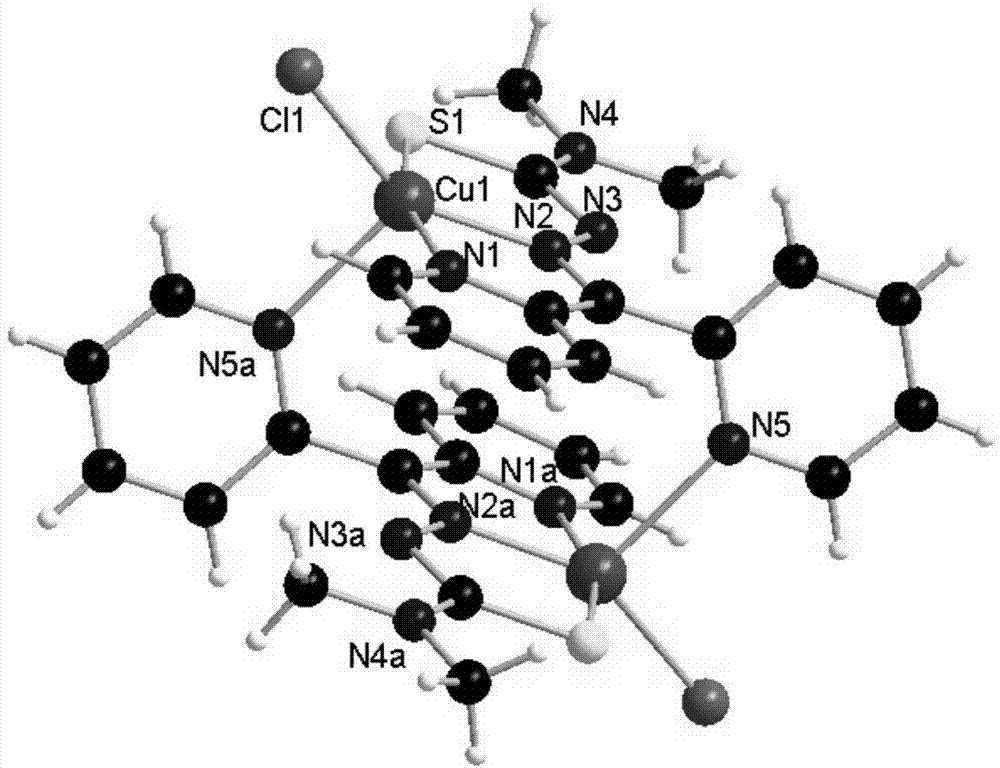
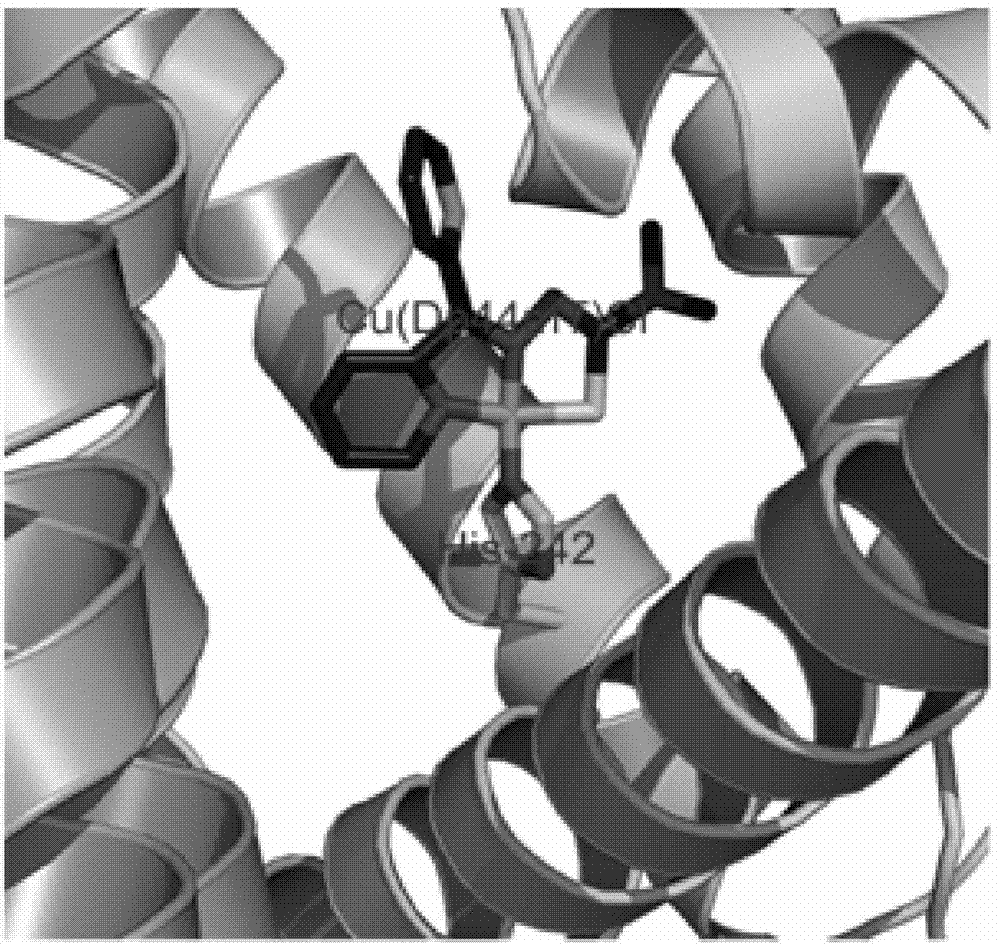
Copper metal complex and compound of copper metal complex and human serum albumin, as well as synthesis methods and application of copper metal complex and compound
A technology of human serum albumin and a synthesis method, applied in the field of copper metal complexes and their complexes with human serum albumin, to achieve the effect of good medicinal value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: [Cu(Dp44mT)Cl] 2 Synthesis of complexes
[0034] The synthetic route is as follows:
[0035]
[0036] The specific synthesis method is:
[0037]1) Dissolve 10mmol of 2-dipyridyl ketone (1833.1mg) in 20ml of ethanol (the concentration of solvent ethanol is 50v / v%), stir at 60°C for 15min to obtain a solution, and add the above solution dropwise to Add 10mmol 4,4'-dimethyl-3-thiosemicarbazide (1191.9mg) in 20ml of ethanol (concentration of solvent ethanol: 50v / v%) solution, reflux and stir at 60°C for 24h to obtain a light yellow precipitate, The light yellow precipitate obtained above was filtered, washed three times with absolute ethanol and ether, and dried to obtain the ligand 2-dipyridyl ketal 4,4'-dimethyl-3-thiosemicarbazide;
[0038] 2) will contain CuCl 2 2H 2 O (170.48mg, 1mmol) 20ml of methanol (the concentration of solvent methanol is 60v / v%) solution, dropwise added to the solution containing 1mmol 2-dipyridyl ketal 4,4'-dimethyl-3-thiosemi...
Embodiment 2
[0051] Example 2: [Cu(Dp44mT)Cl] 2 Synthesis of complexes
[0052] 1) Dissolve 10mmol of 2-dipyridyl ketone (1833.1㎎) in 10ml of methanol (the concentration of solvent methanol is 80v / v%), stir at 50°C for 15min to obtain a solution, and add the above solution dropwise to Add 10mmol 4,4'-dimethyl-3-thiosemicarbazide (1191.9mg) in 20ml of ethanol (concentration of solvent ethanol: 20v / v%) solution, reflux and stir at 80°C for 18h to obtain a light yellow precipitate , after filtering the light yellow precipitate obtained above, washing with water three times, and drying, the ligand 2-dipyridyl ketal 4,4'-dimethyl-3-thiosemicarbazide was obtained;
[0053] 2) will contain CuCl 2 2H 2 O (170.48mg, 1mmol) 20ml of ethanol (concentration of solvent ethanol is 40v / v%) solution, dropwise added to the solution containing 1mmol 2-dipyridyl ketal 4,4'-dimethyl-3-thiosemicarbazide 20ml of ethanol (the concentration of solvent ethanol is 70v / v%) solution, reflux and stir at 50°C for 2h...
Embodiment 3
[0054] Example 3: [Cu(Dp44mT)Cl] 2 Synthesis of complexes
[0055] 1) Dissolve 10mmol of 2-dipyridyl ketone (1833.1㎎) in 10ml of methanol (the concentration of solvent methanol is 80v / v%), stir at 50°C for 15min to obtain a solution, and add the above solution dropwise to Add 10mmol 4,4'-dimethyl-3-thiosemicarbazide (1191.9mg) in 20ml of methanol (concentration of solvent methanol: 60v / v%) solution, stir at 35°C for 48h, and get a pale yellow precipitate The above-mentioned light yellow precipitate was filtered, washed three times with methanol, and dried to obtain the ligand 2-dipyridyl ketal 4,4'-dimethyl-3-thiosemicarbazide;
[0056] 2) will contain CuCl 2 2H 2 O (170.48mg, 1mmol) 20ml of ethanol (the concentration of solvent ethanol is 30v / v%) solution, dropwise added to the solution containing 1mmol 2-dipyridyl ketal 4,4'-dimethyl-3-thiosemicarbazide 20ml ethanol (the concentration of solvent ethanol is 20v / v%) solution, stirred at 40°C for 36h, filtered the reacted s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com