Method for establishing fingerprint spectrum of Jian Ganle preparation
A fingerprint and Jianganle technology, which is applied to establish the fingerprint of Jianganle preparations, detect and establish the quality or authenticity of Jianganle preparations, achieve high resolution, good peak shape, and improve the effect of separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the investigation of chromatographic conditions
[0057] Preparation of the test solution: take an appropriate amount of Jianganle granules, grind and pulverize them, and use 50% ethanol to make a solution with a concentration of 10 mg / mL, and ultrasonicate the obtained solution for 10 minutes to obtain the test solution.
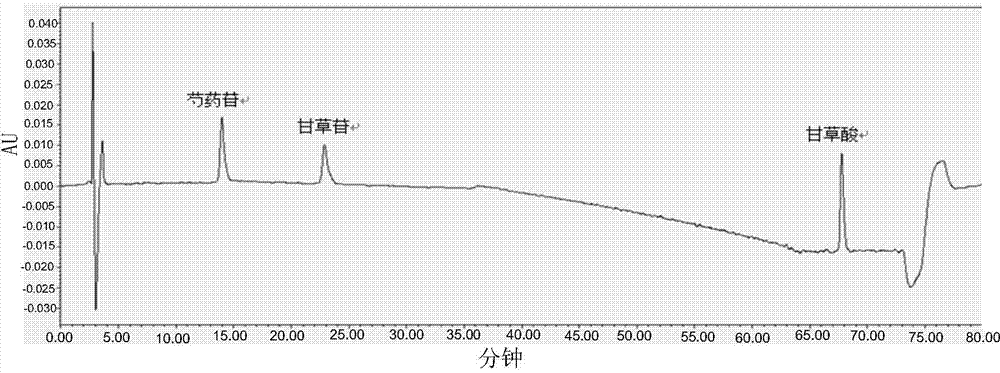
[0058] Preparation of the reference substance solution: Take an appropriate amount of the reference substance of paeoniflorin, weigh it accurately, add methanol to make a solution containing 60 μg of the reference substance of paeoniflorin per 1 ml, and obtain the solution.
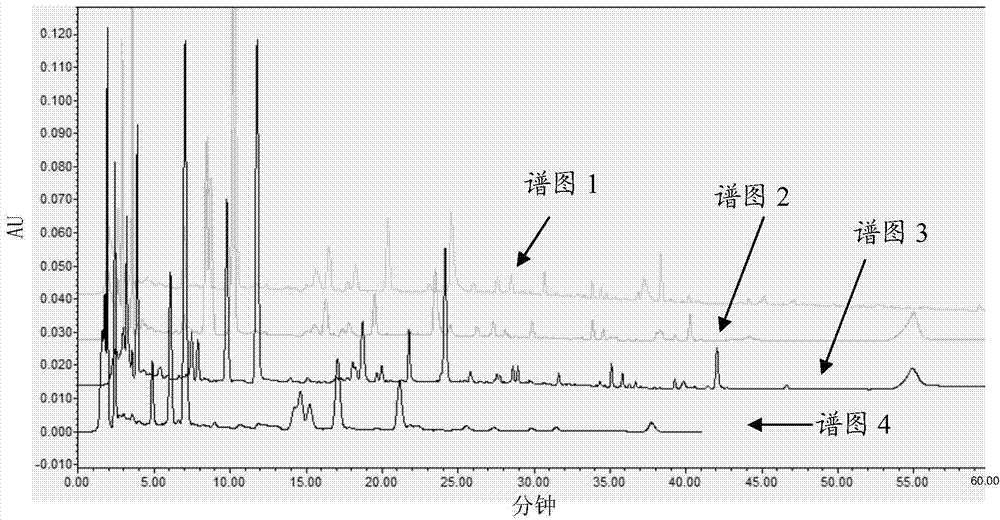
[0059] Test method: inject 10 microliters of the solution to be tested into the high-performance liquid chromatograph, respectively use phosphoric acid-acetonitrile system (that is, phosphoric acid as mobile phase B and acetonitrile as mobile phase A), formic acid-acetonitrile system, phosphoric acid-methanol system, Water-acetonitrile system, water-methanol system...
Embodiment 2
[0073] Embodiment 2: the investigation of need testing solution preparation:
[0074] 1. Investigation of extraction solvent
[0075] Take an appropriate amount of Jianganle granule powder, add methanol, ethanol, 50% methanol, and 50% ethanol respectively to make a solution with a concentration of about 10 mg / mL, and ultrasonicate the obtained solution for 30 minutes, and after cooling, dilute to volume and filter. That is, different test solutions were obtained.
[0076] 10 microliters of the above-mentioned preparations were injected into the high-performance liquid chromatograph, and then detected according to the following chromatographic conditions:
[0077] Octadecylsilane bonded silica gel is used as filler, the elution conditions are as shown in Table 1, the flow rate is 1.0ml / min, the column temperature is 30°C, and the detection wavelength is 232nm; the number of theoretical plates calculated based on the peak of paeoniflorin should not be less than 2000; Waters Sy...
Embodiment 3
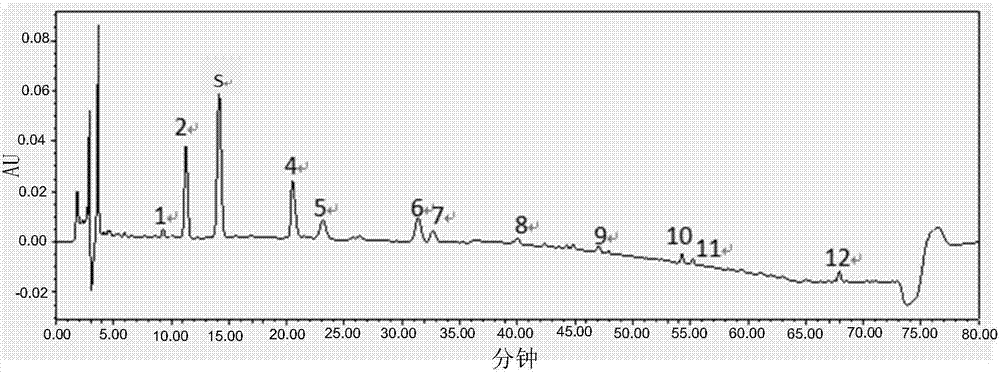
[0083] Example 3: Establishment of fingerprints of Jianganle preparations
[0084] 1. Chromatographic conditions and system suitability test
[0085] Octadecylsilane-bonded silica gel is used as filler; acetonitrile is used as mobile phase A, and 0.05% formic acid aqueous solution is used as mobile phase B. The gradient elution is shown in Table 1. The flow rate is 1.0ml / min, and the column temperature is 30°C. The detection wavelength is 232nm; the number of theoretical plates calculated based on the peak of paeoniflorin should not be less than 2000; Waters Symmetry C18 chromatographic column (4.6mm×250mm, 5μm).
[0086] 2. Preparation of the test solution:
[0087] Take an appropriate amount of Jianganle granule powder, add 50% ethanol to make a solution with a concentration of about 10 mg / ml, and ultrasonicate the obtained solution for 10 minutes, then let it cool down in turn, and constant volume to obtain the test solution.
[0088] 3. Preparation of reference solution:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com