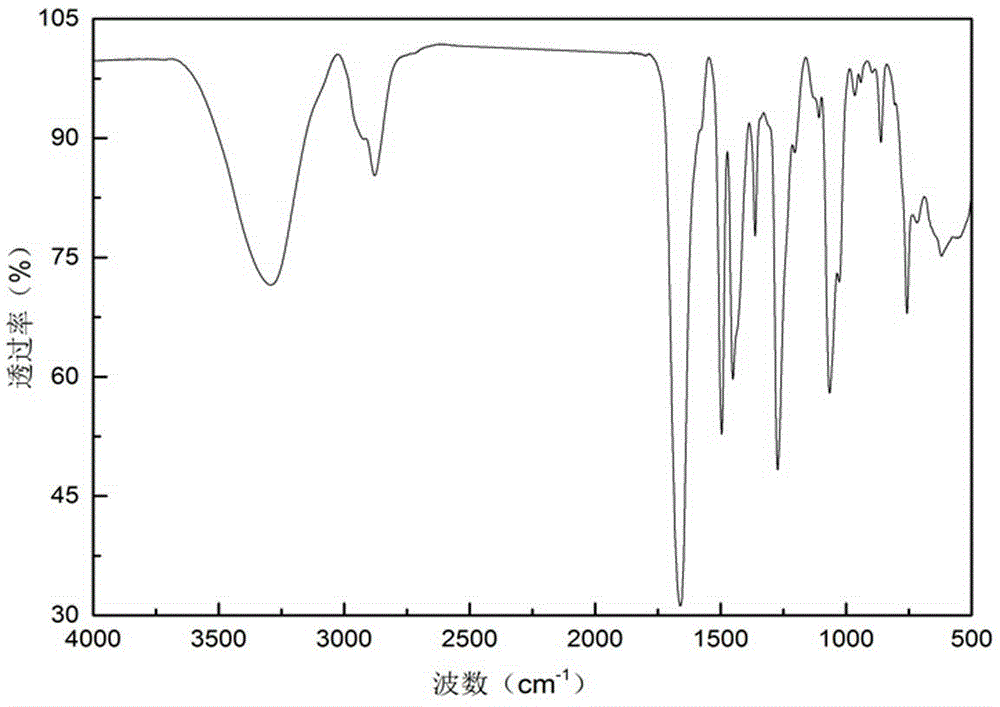
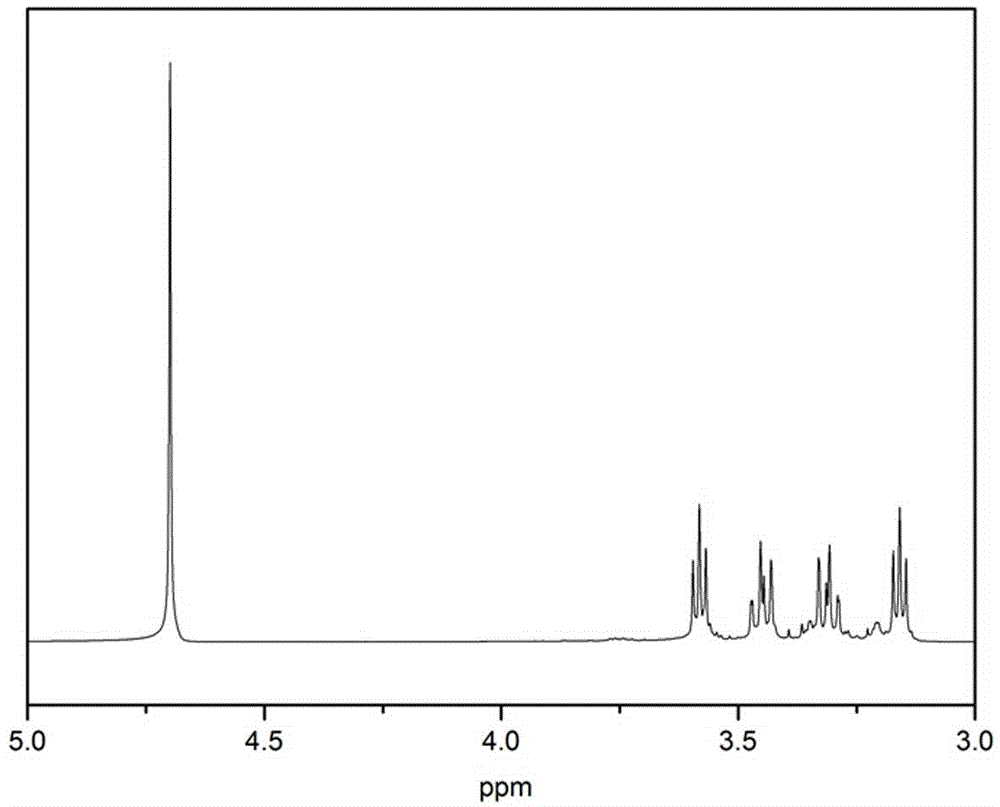
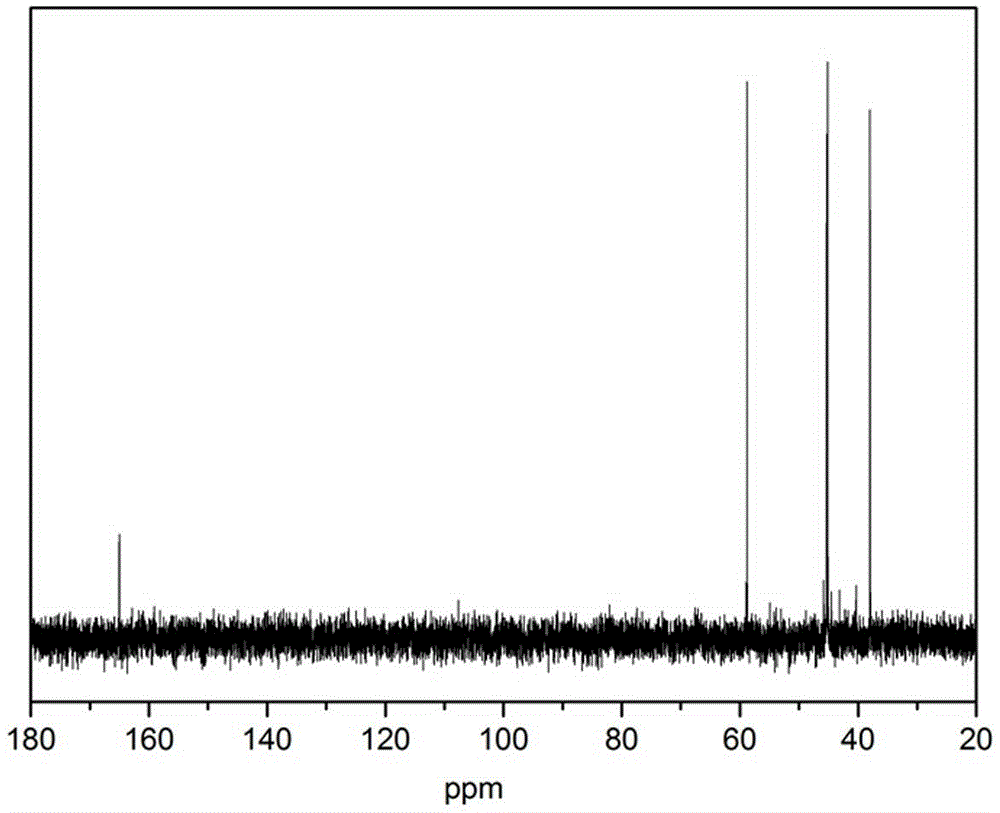
Method for preparing 1-(2-ethoxyl)-2-imidazolone by using CO2 as raw material
A technology of imidazolinone and hydroxyethyl, which is applied in the field of preparation of cyclic urea compounds, can solve the problems of harsh reaction conditions and high cost of reaction raw materials, and achieve the effects of low raw material toxicity, simple and reliable execution route, and scientific design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] with CO 2 The method for raw material preparation 1-(2-hydroxyethyl)-2-imidazolidinone, its step is:
[0033] (1) Add 0.3mol ethanolamine, 20mL ethanol and 3mmol K to the autoclave 3 PO 4 ;
[0034] (2) Pre-introduction of CO 2 , and the reaction temperature was gradually raised to 190°C, and the CO in the autoclave was adjusted to 2 Pressure to 6MPa, react for 6h under mechanical stirring;
[0035] (3) After the reaction is completed, the autoclave is cooled to room temperature, the product is taken out, and the solvent and by-product water are removed by rotary distillation;
[0036] (4) The final product 1-(2-hydroxyethyl)-2-imidazolidinone is obtained by elution through a chromatographic column, and the elution solvent of the chromatographic column is a mixture of ethanol and dichloromethane with a volume ratio of 1:6.
[0037] The yield of the above 1-(2-hydroxyethyl)-2-imidazolidinone was 66.7%.
Embodiment 2
[0039] with CO 2 The method for raw material preparation 1-(2-hydroxyethyl)-2-imidazolidinone, its step is:
[0040] (1) Add 0.3mol ethanolamine, 20mL ethanol and 3mmol Na 3 PO 4 ;
[0041] (2) Pre-introduction of CO 2 , and the reaction temperature was gradually raised to 170°C, and the CO in the autoclave was adjusted 2 Pressure to 6MPa, react for 10h under mechanical stirring;
[0042] (3) After the reaction is completed, the autoclave is cooled to room temperature, the product is taken out, and the solvent and by-product water are removed by rotary distillation;
[0043] (4) The final product 1-(2-hydroxyethyl)-2-imidazolidinone is obtained by elution through a chromatographic column, and the elution solvent of the chromatographic column is a mixture of ethanol and dichloromethane with a volume ratio of 1:4.
[0044] The yield of the above 1-(2-hydroxyethyl)-2-imidazolidinone was 58.2%.
Embodiment 3
[0046] with CO 2 The method for raw material preparation 1-(2-hydroxyethyl)-2-imidazolidinone, its step is:
[0047](1) Add 0.3mol ethanolamine, 20mL ethanol and 3mmol Li 3 PO 4 ;
[0048] (2) Pre-introduction of CO 2 , and the reaction temperature was gradually raised to 170°C, and the CO in the autoclave was adjusted 2 Pressure to 8MPa, react for 6h under mechanical stirring;
[0049] (3) After the reaction is completed, the autoclave is cooled to room temperature, the product is taken out, and the solvent and by-product water are removed by rotary distillation;
[0050] (4) The final product 1-(2-hydroxyethyl)-2-imidazolidinone is obtained by elution through a chromatographic column, and the elution solvent of the chromatographic column is a mixture of ethanol and dichloromethane with a volume ratio of 1:5.
[0051] The yield of the above 1-(2-hydroxyethyl)-2-imidazolidinone was 28.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com