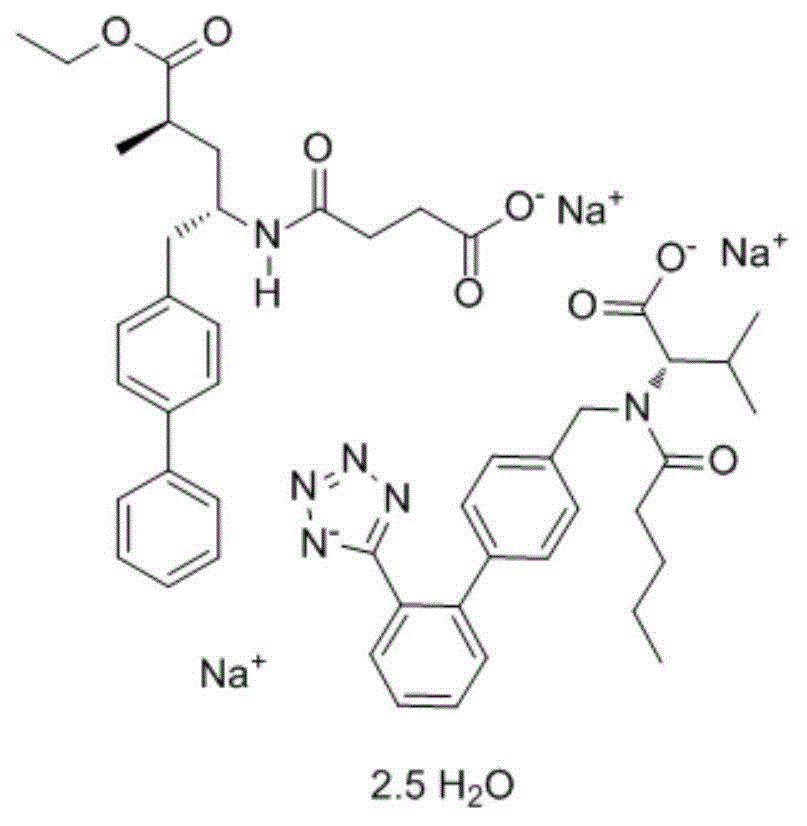
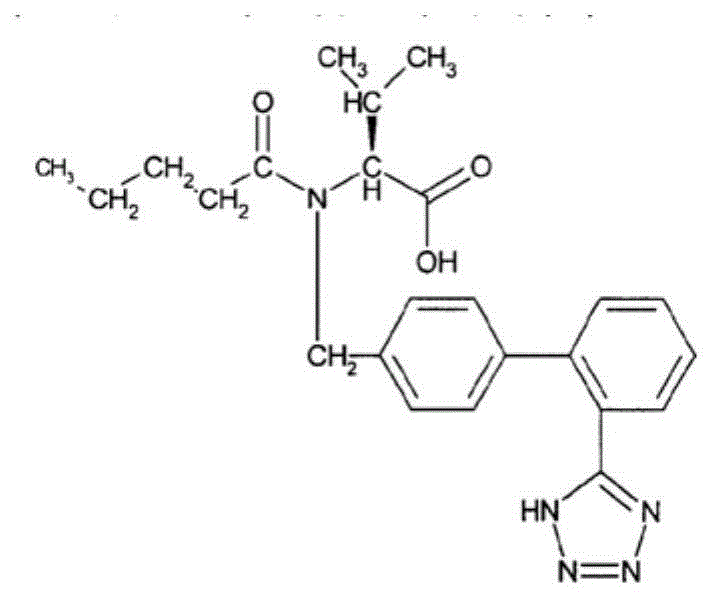
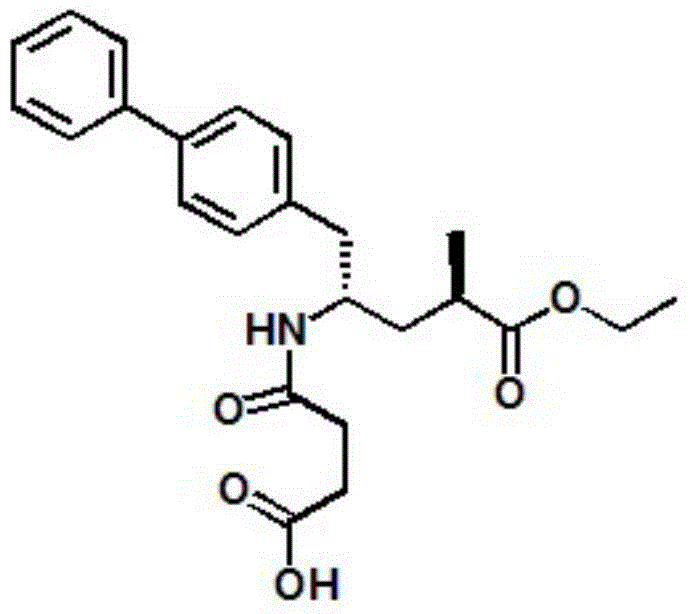
Method for preparing cardiotonic drug LCZ696
A biphenyl, solid technology, applied in the field of preparation of pharmaceutical compounds, can solve the problems of high content, low purity of LCZ696, difficult to remove, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of LCZ696
[0059] step 1,
[0060] Add 10g (2R,4S)-5-([1,1'-biphenyl]-4-yl)-4-amino-2-methylpentanoic acid hydrochloride and 60ml absolute ethanol into the reaction flask, stir Raise the temperature to 65-70°C; keep at 65-70°C, drop 5.6g of thionyl chloride into the reaction bottle under stirring; after the addition, keep stirring at 65-70°C for 3 hours; check the reaction with TCL (if the reaction is not complete, then Add an appropriate amount of thionyl chloride); after the reaction is completed, the solvent is evaporated under reduced pressure, and the temperature is 50°C, and steamed until no liquid flows out; 100ml of n-heptane is added to the residue, stirred and crystallized at room temperature for 1 hour; the temperature is lowered to 0~ Stir and crystallize at 10°C for 2 hours; filter, rinse the filter cake with 50ml of n-heptane, and dry in vacuum for 4 hours at a temperature of 50-55°C; obtain 10.8g of (2R,4S)-5-([1,1'- Biphenyl]-4-yl)-4-amino...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com