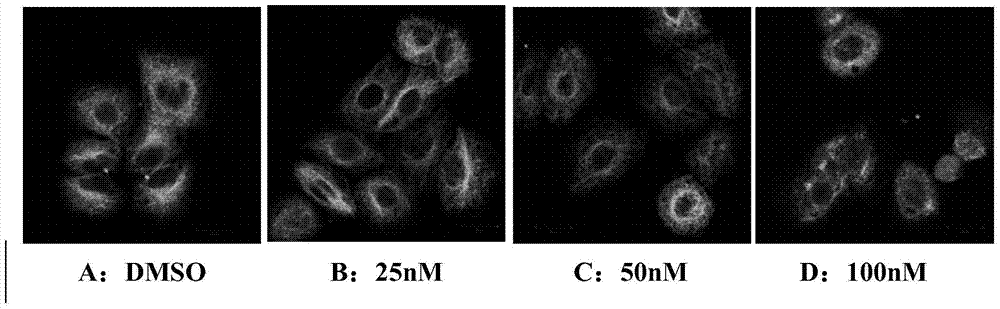
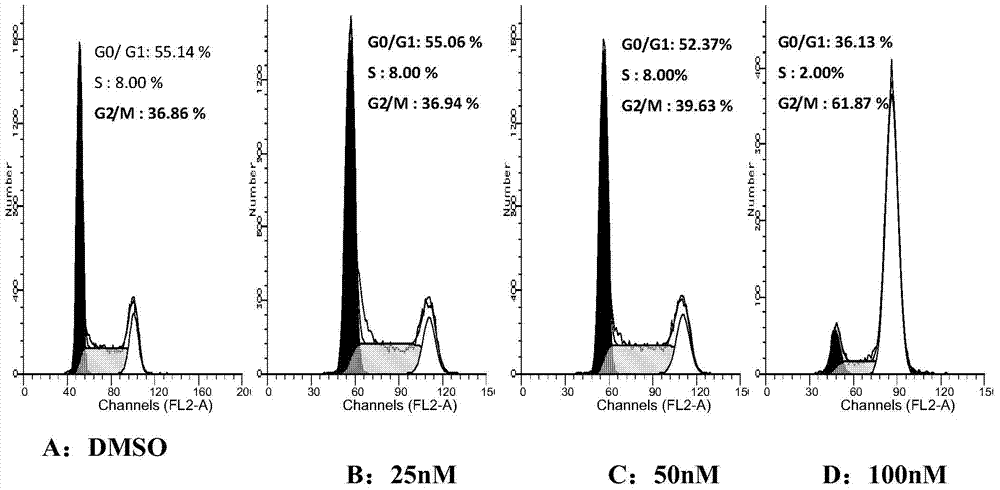
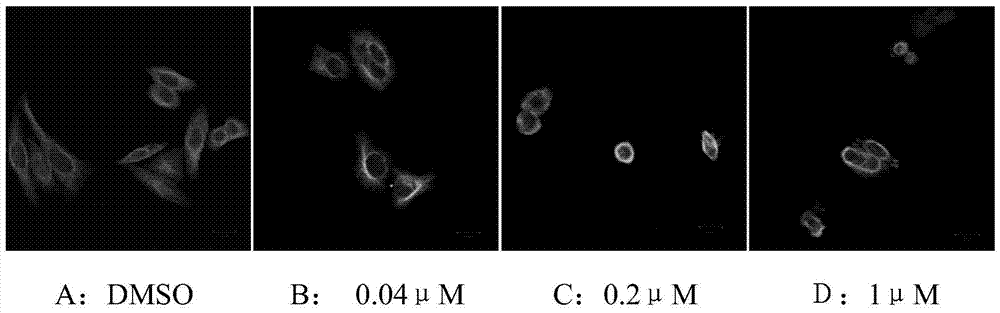
5-arylphenol-2 alkyl substituted urea benzimidazole compounds and their applications
A technology of benzimidazole and arylphenol, which is applied in the field of chemical medicine, can solve the problems of complex chemical structure and poor solubility, and achieve the effects of wide source of raw materials, low cost and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Synthesis of 1-(5-phenoxy-1H-benzimidazole-2)-3-methylurea
[0073] Step 12 - Synthesis of nitro-5-phenoxyaniline
[0074]
[0075] Dissolve 0.9mL (10mmol) of phenol in 10mL of N,N-dimethylformamide, add 1.4g (10mmol) of anhydrous potassium carbonate, and stir at 100°C for 1h. Subsequently, 0.8 g (5 mmol) of 5-fluoro-2-nitroaniline was added and reacted at 100° C. for about 6 h. The reaction was tracked by TLC, and it was found that the raw material had reacted completely. After being cooled to room temperature, it was poured into ice water, and a precipitate was precipitated. It was suction-filtered with a Buchner funnel to obtain 1.1 g of crude product 2-nitro-5-phenoxyaniline, and the yield was 98.5%. The crude product was directly used for Next reaction.
[0076] Step 24 - Synthesis of phenoxy-1,2-phenylenediamine
[0077]
[0078] Dissolve 1.1g (4.9mmol) of 2-nitro-5-phenoxyaniline synthesized in the previous step in 10mL of ethanol, add 0.03g (0.24mmol) o...
Embodiment 2
[0086] Synthesis of 1-(5-phenoxy-1H-benzimidazole-2)-3-ethylurea
[0087]
[0088] Weigh 0.6g (2.1mmol) of methyl-5-benzyloxy-1H-benzimidazole-2-carbamate into 5mL acetonitrile, 0.54mL (4.2mmol) 70% ethylamine aqueous solution, microwave 140 After reacting at ℃ for 2 hours, it was poured into ice water, and a precipitate was precipitated. It was filtered under reduced pressure to obtain 0.32 g of the product 1-(5-phenoxy-1H-benzimidazole-2)-3-ethylurea, with a yield of 51.0%. 1 HNMR (400MHz, DMSO-d6) δ11.53(s,1H),9.85(s,1H),7.36-7.31(m,3H),7.20(s,1H),7.06-7.03(m,2H),6.91 (d, J=8.4Hz, 2H), 6.75(d, J=8.4Hz, 1H), 3.22-3.18(m, 2H), 1.10(t, J=7.2Hz, 3H). HRMS (ESI), [M+H] + : 297.1347.
Embodiment 3
[0090] Synthesis of 1-(5-(2-chlorophenoxy)-1H-benzimidazole-2)-3-methylurea
[0091]
[0092] Using 2-chlorophenol as the starting material, the procedure is the same as that of 1-(5-phenoxy-1H-benzimidazole-2)-3-methylurea, and the total yield is 8.5%. Among them, the method of nitro reduction is iron powder reduction, and the specific operation is as follows: add 1.4g (24.6mmol) iron powder to 2mL water, drop into concentrated hydrochloric acid 0.5mL, and reflux at 100°C for 1h. Add 1.3 g (4.9 mmol) of 2-nitro-5-(2-chlorophenoxy)aniline dissolved in 6 mL of ethanol, and react overnight. Suction filtration while hot, adjust the pH of the filtrate to neutral with a saturated solution of sodium bicarbonate, there is precipitation, suction filtration under reduced pressure, spin the filtrate to dry the solvent, and column chromatography to obtain 1.0g of the product 1-(5-(2-chlorophenoxy Base)-1H-benzimidazole-2)-3-methylurea, the yield was 87.2%. 1 HNMR (400MHz, DMSO-d6) δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com