Diaryl ether derivatives as well as preparation method and application thereof
A kind of derivative, the technology of diaryl ether, applied in the application of related medicines, diaryl ether derivatives and the field of preparation thereof, to achieve the effects of small cytotoxicity, enhanced binding force, and strong anti-HIV-1 virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
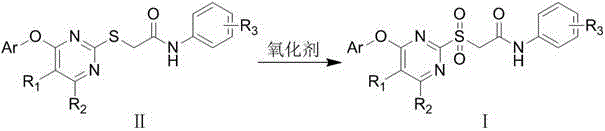
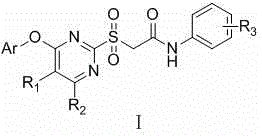
[0024] Embodiment 1: the synthesis of final product I
[0025] At 20~40℃, add thioether II into the solvent, stir to dissolve, then add oxidant, and stir for 5-10h. TLC showed the reaction was complete. It was washed successively with saturated sodium sulfite solution, saturated sodium carbonate solution, water and saturated brine, and the organic phase was dried over anhydrous sodium sulfate overnight. Filtration, concentration, and recrystallization from toluene gave the desired solid.
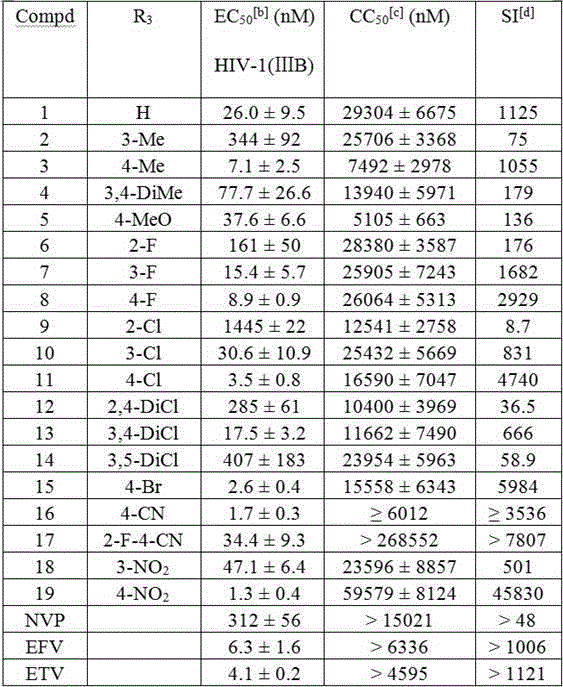
[0026] The target compounds were prepared by the above method with different thioethers II, and some results are as follows:
[0027] At room temperature, add 2-{[4-(4-cyano-2,6-dimethylphenol)pyrimidin-2-yl]thio}acetanilide (5.53 mmol) into 60 mL of dichloromethane, stir Dissolved, then added m-chloroperoxybenzoic acid (12.16 mmol), stirred for 9h. TLC showed the reaction was complete. Wash with saturated sodium sulfite solution (20 mL × 2), saturated sodium carbonate solution (20 mL ×...
Embodiment 2
[0066] Embodiment 2: anti-HIV biological activity test
[0067] The anti-HIV virus activity at the cell level in vitro was determined by the Rega Institute of Pharmacy at Katholleke University in Belgium, mainly including: inhibitory activity and cytotoxicity to HIV-infected MT-4 cells. The method is as follows: make the compound in HIV-infected MT-4 cells, at different times of HIV infection, use the MTT method to measure the protective effect of the drug on HIV-induced cytopathy, and calculate that 50% of the cells are free from HIV-induced cytopathy half effective concentration EC 50 , the toxicity assay was carried out in parallel with the anti-HIV activity experiment, also in MT-4 cell culture, the concentration that caused 50% of uninfected cells to undergo cytopathic changes was determined by MTT method (CC 50 ), and calculate the selectivity index SI = CC 50 / EC 50 .
[0068] Materials and Methods:
[0069] The anti-HIV activity of each compound is monitored by th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com