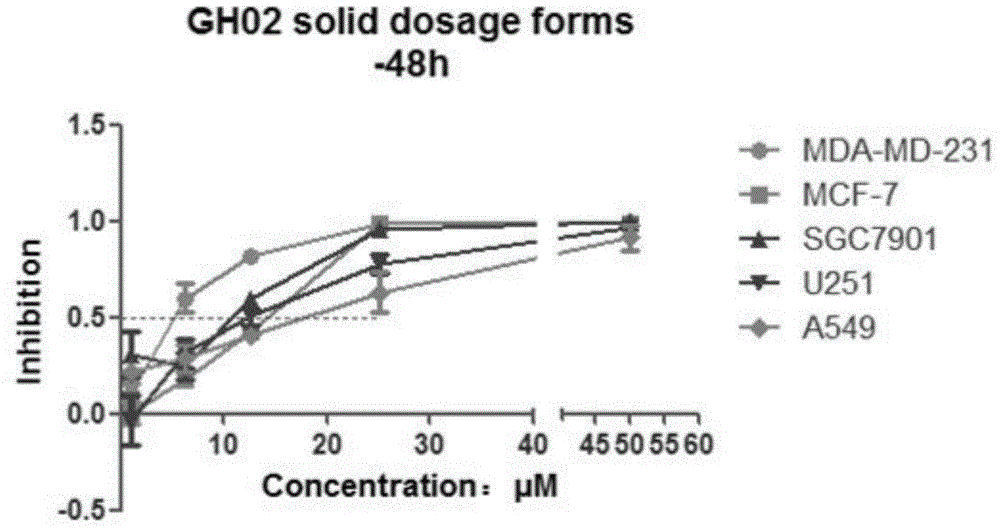
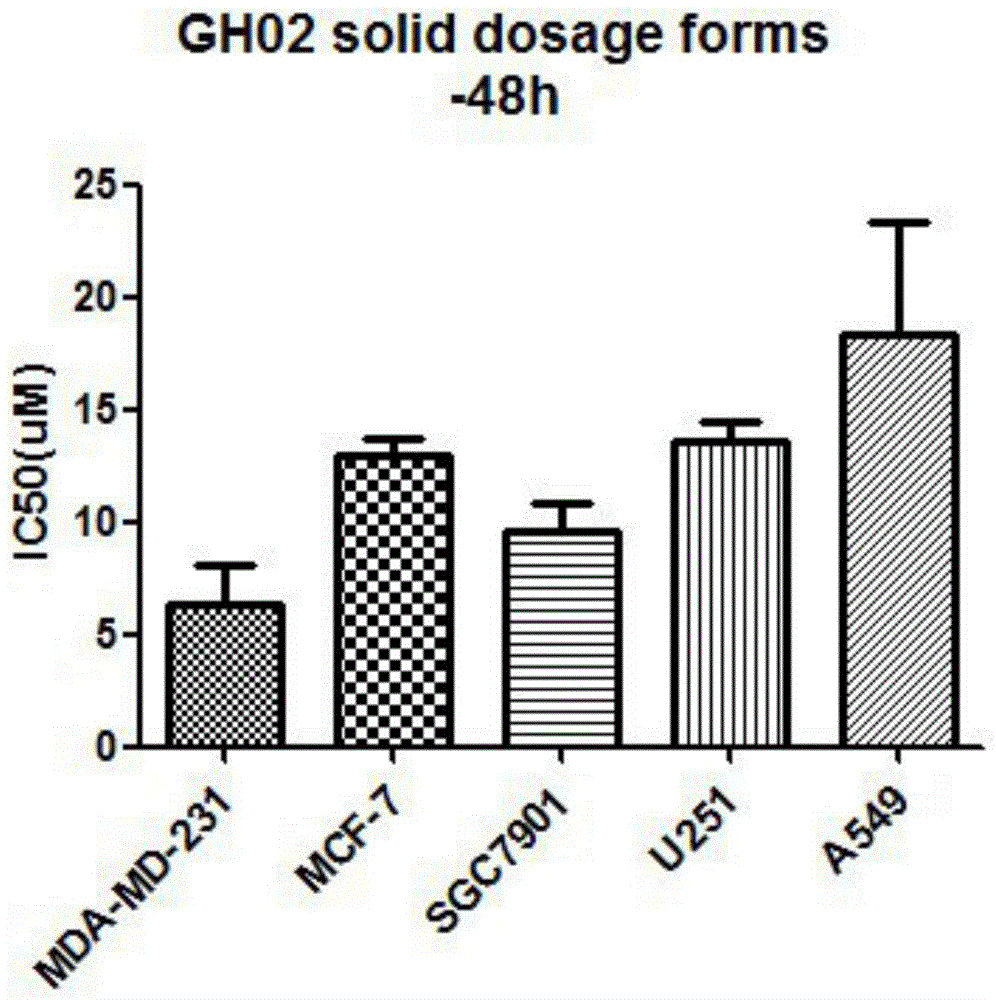
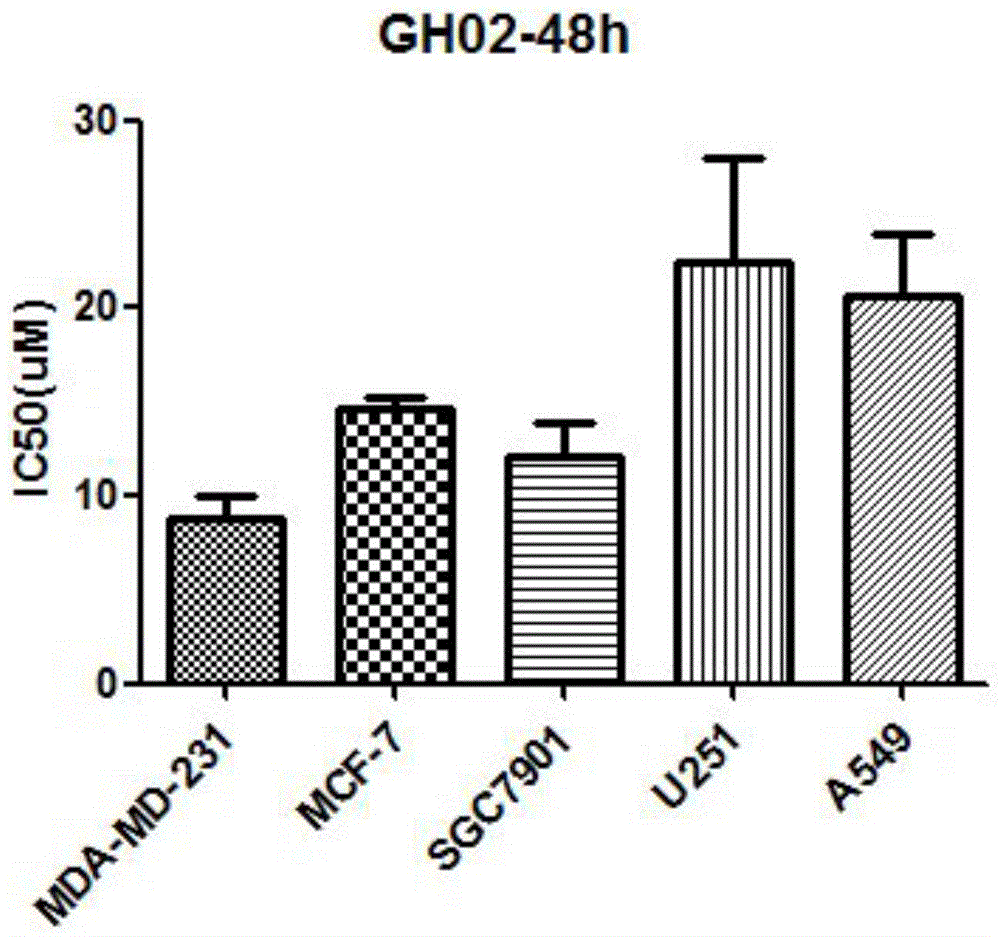
Glaucocalyxin derivative, and pharmaceutically acceptable salt dosage forms thereof
A technology of indigo calyxine and derivatives, applied in the field of chemical medicine, can solve the problems of poor water solubility, low polarity of calyxine, and achieve the effects of low cost, good oral bioavailability and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This example relates to the dosage forms of cerulein A derivatives and their pharmaceutically acceptable salt injections. The injections include: injections, sterile powders for injections, and concentrated solutions for injections. The injections are used for subcutaneous injections and intramuscular injections. , intravenous injection, intravenous drip.
[0041] The above-mentioned cyanine A derivative salt is obtained by dissolving the cyanine A derivative in an organic solvent to form a solution, and then reacting with an acid to form a salt to control the pH value of the solution to obtain a cyanine A derivative salt. The above-mentioned acid includes Organic acids and inorganic acids, the above-mentioned inorganic acids include any one of the following acids: hypoiodous acid, hypochlorous acid, hypobromous acid, iodic acid, perchloric acid, peroxydisulfuric acid, peroxydicarbonic acid, percarbonic acid, pyrophosphoric acid , pyrosulfuric acid, pyrosulfurous acid, ...
Embodiment 2
[0050] Disclosed in embodiment 2 is a method for preparing cyanine A derivatives and pharmaceutically acceptable salt injection thereof. In the prescription of this embodiment, dimethylamino cyanine A hydrochloride is used as the main drug, and the prescription is 30㎎ main drug is dissolved in 10ml solvent.
[0051] The formula for preparing the injection in this embodiment is consistent with the formula in Example 1, as shown in Table 1, and the preparation method in Example 2 is also consistent as in Example 1, except that the pH in Example 2 The value is not the same as the molar concentration of the osmotic pressure regulator.
[0052] The specific method is: dissolve sodium chloride and sodium metabisulfite with one-third of the total volume of water for injection, then dissolve dimethylaminocyanine A hydrochloride with an appropriate amount of 0.01mol / L hydrochloric acid, and then dissolve the dissolved Add the mixture of dimethylaminocyanine A hydrochloride to the sodi...
Embodiment 3
[0055] Disclosed in embodiment 3 is a method for preparing cyanine A derivatives and pharmaceutically acceptable salt injection thereof. In the prescription of this embodiment, dimethylamino cyanine A hydrochloride is used as the main drug, and the prescription is 30㎎ main drug is dissolved in 10ml solvent.
[0056] The formula for preparing the injection in this embodiment is consistent with the formula in Example 1, as shown in Table 1, and the preparation method in Example 2 is also consistent as in Example 1, except that the pH in Example 2 The value is not the same as the molar concentration of the osmotic pressure regulator.
[0057] The specific method is: dissolve sodium chloride and sodium metabisulfite with one-third of the total volume of water for injection, then dissolve dimethylaminocyanine A hydrochloride with an appropriate amount of 0.01mol / L hydrochloric acid, and then dissolve the dissolved Add the mixture of dimethylaminocyanine A hydrochloride to the sodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com