2-perfluoroalkyl indole derivative and synthesis method thereof
A technology of perfluoroalkyl indole derivatives and perfluoroalkyl, applied in organic chemistry and other fields, can solve problems such as inability to reflect diversity and cumbersome steps, and achieve unique chemical stability, physiological stability, and regioselectivity High, extended selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: 2-trifluoromethyl-1 H - the preparation of indole-3-carboxylic acid methyl ester, the structure of this compound is:
[0021]
[0022] Molecular formula: C 11 h 8 f 3 NO 2
[0023] Chinese name: 2-trifluoromethyl-1 H -Methyl indole-3-carboxylate
[0024] English name: methyl 2-(trifluoromethyl)-1 H -indole-3-carboxylate
[0025] Molecular weight: 243.05
[0026] Melting point: 135.6°C-136.5°C
[0027] Appearance: white solid
[0028] Proton NMR spectrum (500MHz, CDCl 3, Internal standard: TMS): 3.99 (s, 3H, OCH3), 7.32-7.35 (m, 1H, ArH), 7.37-7.40 (m, 1H, ArH), 7.48 (d, J = 8.1 Hz, 1H, ArH) , 8.26 (d, J = 8.1 Hz, 1H, ArH), 9.29 (br s, 1H, NH) ppm;
[0029] NMR fluorine spectrum (470MHz, CDCl 3 , internal standard: C 6 f 6 ): -59.85 (s, CF 3 ) ppm.
Embodiment 2
[0030] Example 2: 5-methoxy-2-trifluoromethyl-1 H - the preparation of indole-3-carboxylic acid methyl ester, the structure of this compound is:
[0031]
[0032] Molecular formula: C 12 h 10 f 3 NO 3
[0033] Chinese name: 5-methoxy-2-trifluoromethyl-1 H -Methyl indole-3-carboxylate
[0034] English name: methyl 5-methoxy-2-(trifluoromethyl)-1 H -indole-3-carboxylate
[0035] Molecular weight: 273.06
[0036] Melting point: 157.4°C-158.3°C
[0037] Appearance: white solid
[0038] Proton NMR spectrum (500MHz, CDCl 3, Internal standard: TMS): 3.87 (s, 3H), 3.97 (s, 3H), 7.01-7.03 (m, 1H), 7.35 (d, J = 9.0 Hz, 1H), 7.68 (d, J = 2.0 Hz, 1H), 9.28 (br s, 1H) ppm;
[0039] NMR fluorine spectrum (470MHz, CDCl 3 , internal standard: C 6 f 6 ): -59.86 (s, CF 3 ) ppm.
[0040] (1) A 2-perfluoroalkylindole derivative, characterized in that the structure of the compound is:
[0041]
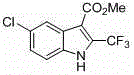
[0042] 5-Chloro-2-trifluoromethyl-1 H -Methyl indole-3-carboxylate
[0043]...
example 1
[0367] Example 1: Preparation of 2-trifluoromethyl-1H-indole-3-carboxylic acid methyl ester
[0368] Add aniline (46.5 mg, 0.5 mmol), Pd 2 (dba) 3 (0.05g, 10 mol%), NaHCO 3 (12.5 mg, 30 mol%), pivalic acid 0.44g and a stirring magnet, after replacing oxygen, add N,N-dimethylacetamide 2 ml and methyl trifluoromethylynoate (91.2 mg, 0.6 mmol ). Stirring at 120°C for 12h, after the completion of the reaction by TLC, column chromatography gave a white solid which was 2-trifluoromethyl-1 H - methyl indole-3-carboxylate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com