Water-soluble sarafloxacin mesylate and preparation method thereof
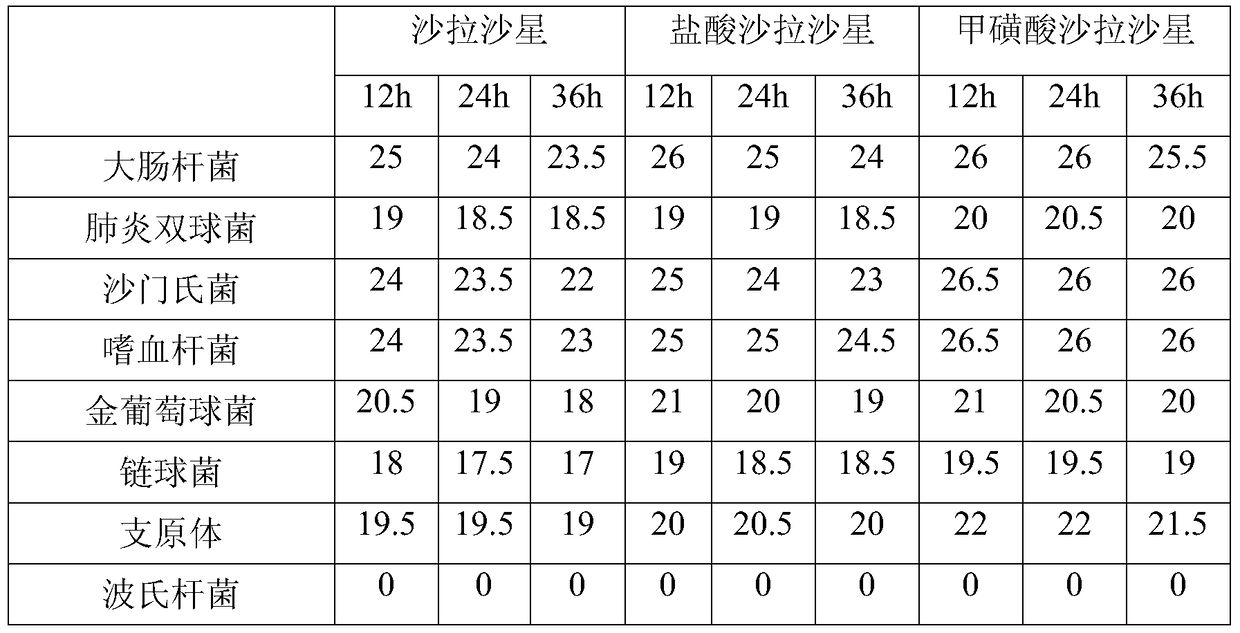
The technology of salafloxacin and methanesulfonic acid is applied in the fields of sulfonate preparation, resistance to vector-borne diseases, organic chemistry, etc. Strong antibacterial activity and broad antibacterial spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preparation of embodiment 1 sarafloxacin mesylate
[0018] Dissolve sarafloxacin hydrochloride in water, adjust the pH to about 7.5 with sodium hydroxide, precipitate solids, filter, and collect sarafloxacin solids.
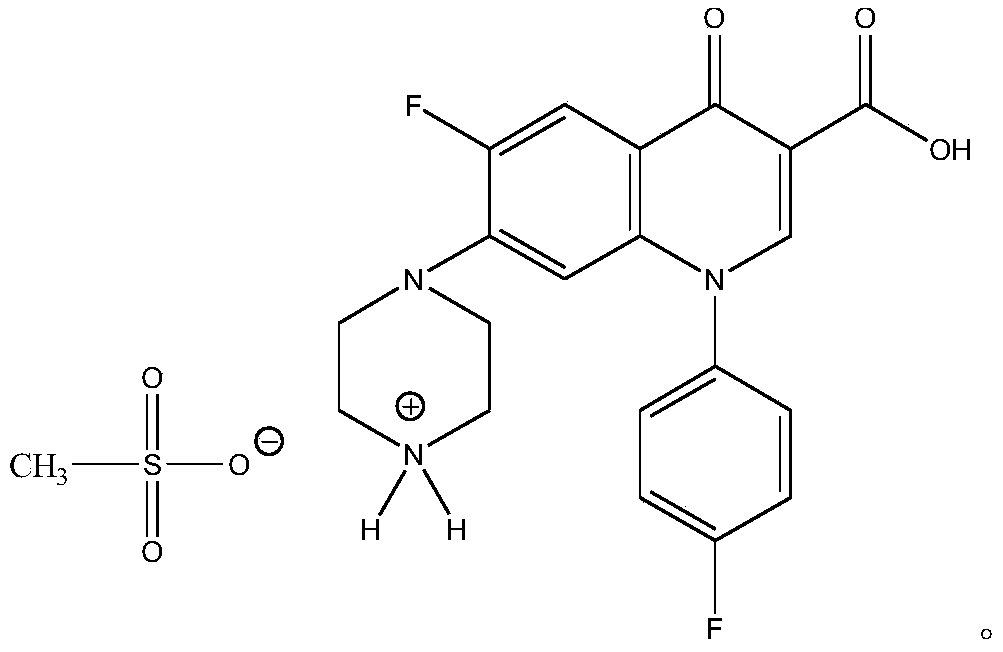
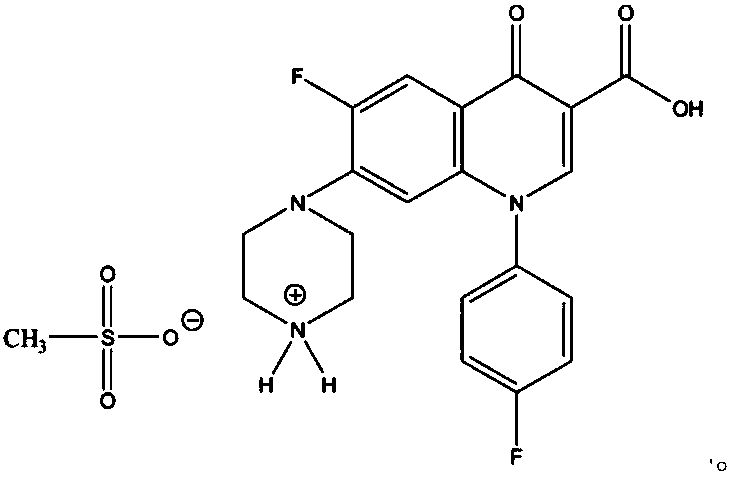
[0019] Dissolve the sarafloxacin (1mol) solid in a mixed solvent of ethanol and water (the weight ratio of ethanol to water is 1:0.05), add methanesulfonic acid whose molar number is 1 times that of sarafloxacin under heating to 80°C, and stir 2.0 hours, cooled for 10 hours to precipitate crystals, collected by filtration, washed with ethanol for 1 to 3 times, and dried in vacuum to obtain the target product. White crystals, yield 92%, fast atom bombardment mass spectrometry MS (FAB): m / e 481.39 (M + ), 385.37 (M + -CH 3 SO 3 H), consistent with the molecular weight theoretically calculated value of target product sarafloxacin mesylate 481.47.
Embodiment 2
[0020] The preparation of embodiment 2 sarafloxacin mesylate
[0021] Dissolve sarafloxacin hydrochloride in water, adjust the pH to about 7.5 with sodium hydroxide, precipitate solids, filter, and collect sarafloxacin solids.
[0022] Dissolve sarafloxacin (1mol) solid in a mixed solvent of isopropanol and water (the weight ratio of isopropanol to water is 1:0.1), add formazan with 1.2 times the mole number of sarafloxacin under the condition of heating to 90°C Sulfonic acid, stirred for 0.5 hours, cooled for 20 hours to precipitate crystals, collected by filtration, washed with acetonitrile for 1 to 3 times, and dried in vacuum to obtain the target product. White crystals, yield 91.5%, MS (FAB): m / e 481.43 (M + ), 385.28 (M + -CH 3 SO 3 H), consistent with the molecular weight theoretically calculated value of target product sarafloxacin mesylate 481.47.
Embodiment 3
[0023] The preparation of embodiment 3 sarafloxacin mesylate
[0024] Dissolve sarafloxacin hydrochloride in water, adjust the pH to about 7.5 with sodium hydroxide, precipitate solids, filter, and collect sarafloxacin solids.
[0025] Dissolve the solid of sarafloxacin (1mol) in acetonitrile solvent, add methanesulfonic acid whose molar number is 1.5 times that of sarafloxacin under heating to 65°C, stir for 0.5 hours, cool for 20 hours to precipitate crystals, collect the crystals by filtration, and use Wash with isopropanol for 1 to 3 times, and dry in vacuum to obtain the target product. White crystals, yield 88.4%, MS (FAB): m / e 482.02 (M + ), 385.44 (M + -CH 3 SO 3 H), consistent with the molecular weight theoretically calculated value of target product sarafloxacin mesylate 481.47.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com