Ezetimibe intermediate and synthesis method of ezetimibe
A technology of ezetimibe and synthesis method, applied in organic chemistry, bulk chemical production, etc., can solve the problems of low yield and purity, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
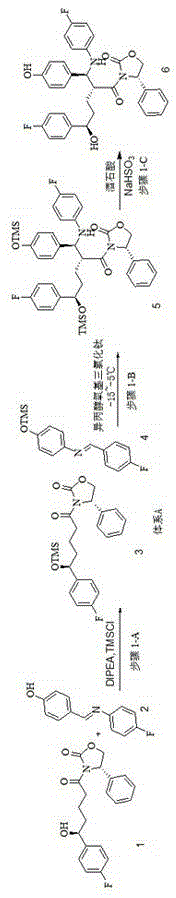
[0056] Embodiment 1: Preparation of ezetimibe intermediate;
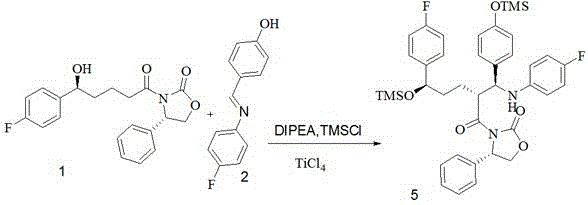
[0057] Add the above-mentioned compound 1 (53.85Kg), compound 2 (64.86Kg), dichloromethane (372.4kg) into a 1000L enamel reaction kettle, control the temperature at -5°C, add DIPEA (139L) dropwise, and complete the dropwise addition within 20min. Start to add trimethylchlorosilane (72.5 L) dropwise, and half an hour later, the dropwise addition is completed, and the stirring is continued for 3 hours, which is recorded as reaction solution A.
[0058] Add titanium tetrachloride (19.83L) into a 200L reaction kettle containing 85L of dichloromethane, cool down to -15°C, add titanium tetraisopropoxide (18.01L), stir for 20min, milky white suspension, cool down to -10°C °C, transfer the above system to a 3000L enamel reaction kettle, add the reaction solution A dropwise, the temperature does not exceed -25 °C, the dropwise addition is completed after 1h, and vigorously stirred for 3h. Add 500L of isopropanol dropwise, a...
Embodiment 2
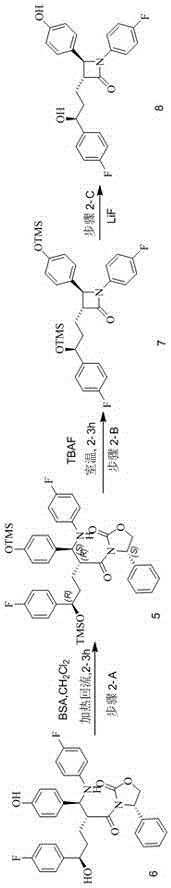
[0060] Embodiment 2: Preparation of ezetimibe;
[0061] Compound 6 (47.7Kg), dichloromethane (250L), and BSA (81.6L) were put into a 1000L enamel reaction kettle, heated to reflux, and stirred for 3h. Return to normal temperature, add TBAF (1.4Kg), the turbidity becomes light yellow and transparent, and react for 2 hours. Compound 5 disappeared and transformed into Compound 7. After the reaction was completed, the organic phase was distilled off, and 135 L of isopropanol and lithium fluoride (2.1 kg) were added, and stirred at room temperature for 1 h; the isopropanol was distilled off at 30° C., and 135 L of dichloromethane and 135 L of purified water were added. , transferred to a 500L layered crystallization tank, stirred for 5min, and separated and extracted. Add 170 L of water to the organic phase, stir at 0°C under temperature control, solid precipitates, filter after 30 min, and wash with water to obtain 28.7 Kg. Dry at 40°C for 12 hours to obtain 31.5Kg of crude prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com