Synthesis method of allyl oxetane compound for ultraviolet light curing
A technology of allyl oxetane and hydroxymethyl oxetane, which is applied in the field of organic chemistry and new materials, can solve the problem of poor mechanical and thermal properties, unsatisfactory curing speed, and large volume shrinkage and other problems, to achieve the effect of simple and easy feeding process, high utilization rate of reactor, and simple feeding process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Add 23.2 g (0.2 mol) 3-ethyl-3-hydroxymethyl oxetane, 48.4 g (0.4 mol) allyl Bromine, 50 g (50%) potassium hydroxide aqueous solution, adjust the magnetic rotary stirrer to make it stir vigorously, then add 1.0 g tetrabutylammonium bromide, keep the reaction at about 0 °C, react for 28 hours, and the reaction ends. 32.5 g of the organic phase was separated, and the content of the product 3-ethyl-3-allylmethoxyxetane in the organic phase was analyzed by gas chromatography to be 83%. The reaction yield reached 86.5%.
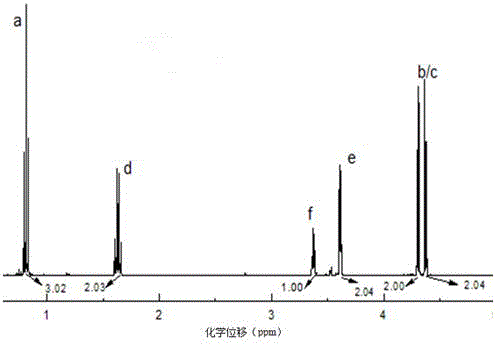
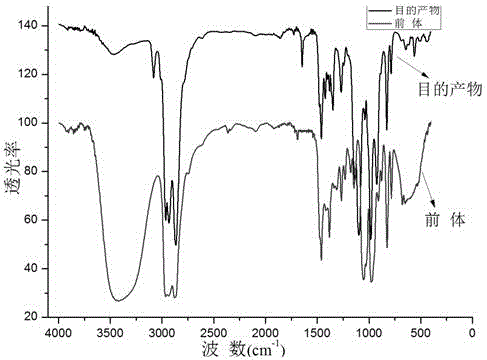
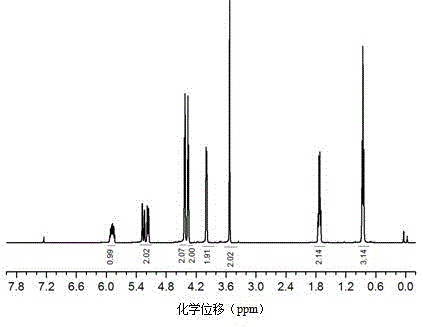
[0038] Raw material 3-ethyl-3-hydroxymethyl oxetane in embodiment 1 and synthetic product 1 H-NMR and FT-IR spectrum see figure 1 , figure 2 , image 3 .
Embodiment 2
[0040] Add 23.2 g (0.2 mol) 3-ethyl-3-hydroxymethyl oxetane, 36.3 g (0.3 mol) allyl Bromine, 50 g (40%) sodium hydroxide aqueous solution, adjust the magnetic stirrer to make it stir vigorously, then add 1.0 g tetraphenylphosphonium chloride, keep the reaction at about 0 ° C, react for 28 hours, and the reaction is over. 31.4 g of the organic phase was separated, and the content of the product 3-ethyl-3-allylmethoxyxetane in the organic phase was analyzed by gas chromatography to be 75%. The reaction yield reached 75.5%.
Embodiment 3
[0042] Add 20.4 g (0.2 mol) 3-methyl-3-hydroxymethyl oxetane, 48.4 g (0.4 mol) allyl Bromine, 50 g (50%) potassium hydroxide aqueous solution, adjust the magnetic stirrer to make it stir vigorously, then add 1.0 g tetrabutylammonium bromide, keep the reaction at about 0 ° C, react for 29 hours, and the reaction ends. 31.6 g of the organic phase was separated, and the content of the product 3-methyl-3-allylmethoxyxetane in the organic phase was analyzed by gas chromatography to be 80%. The reaction yield reached 89%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com