Absorbable biliary duct stent and preparation method thereof
A biliary tract and annular support technology, which is applied in the field of medical devices, can solve the problems of limited use, inflammatory reaction caused by permanent stent placement, poor expansion coefficient and mechanical properties, so as to avoid bile duct obstruction and treat benign and malignant hyperplasia and stricture of bile duct Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
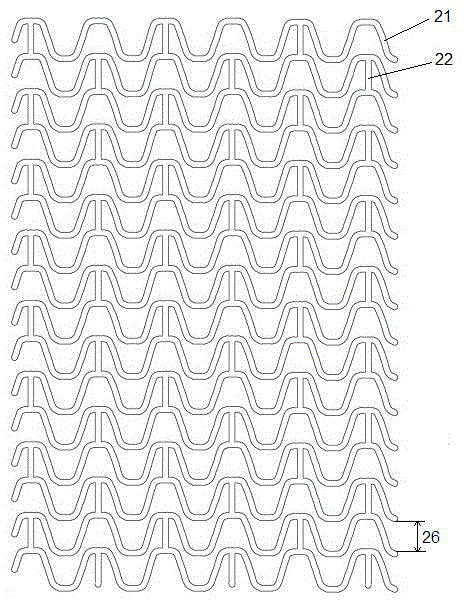
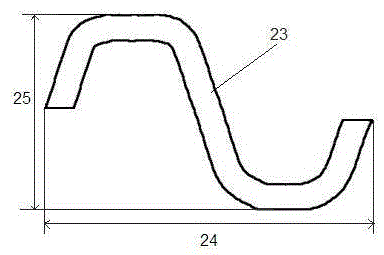
[0027] An absorbable biliary tract stent comprises a stent main body with a tubular filament mesh structure and a drug layer coated on the surface of the stent main body. The main body of the stent is laser-engraved from an absorbable polymer material tube, and the bioabsorbable polymer material is one of polylactic acid and polyglycolide polymer materials, or a mixture of two polymer materials, or A copolymer of synthetic monomers of two polymeric materials. The drug layer includes one of racemic polylactic acid and polylactic acid-polyglycolide block polymer, and anti-biliary tract tumor drugs and / or anti-gallstone drugs. In a specific embodiment, the anti-biliary tract tumor drug is one or more of 5-fluorouracil, cisplatin, and doxorubicin, and the anti-gallstone drug is one or more of sodium cholate and edetate disodium.
[0028] Such as figure 1 with figure 2 As shown, the filament network structure of the above-mentioned stent body includes multiple sets of ring-shap...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com