Patents
Literature
76 results about "Biliary stents" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
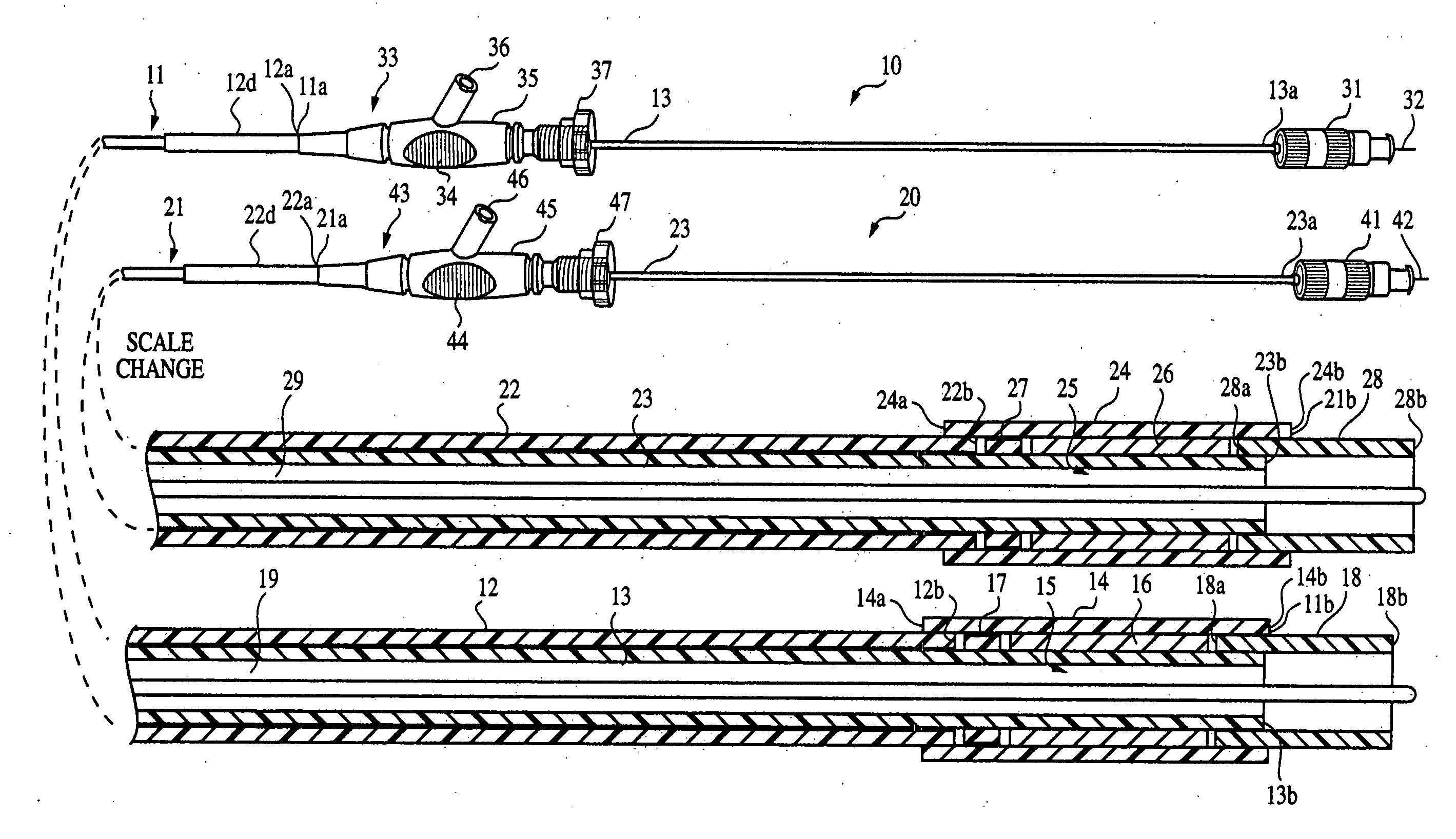
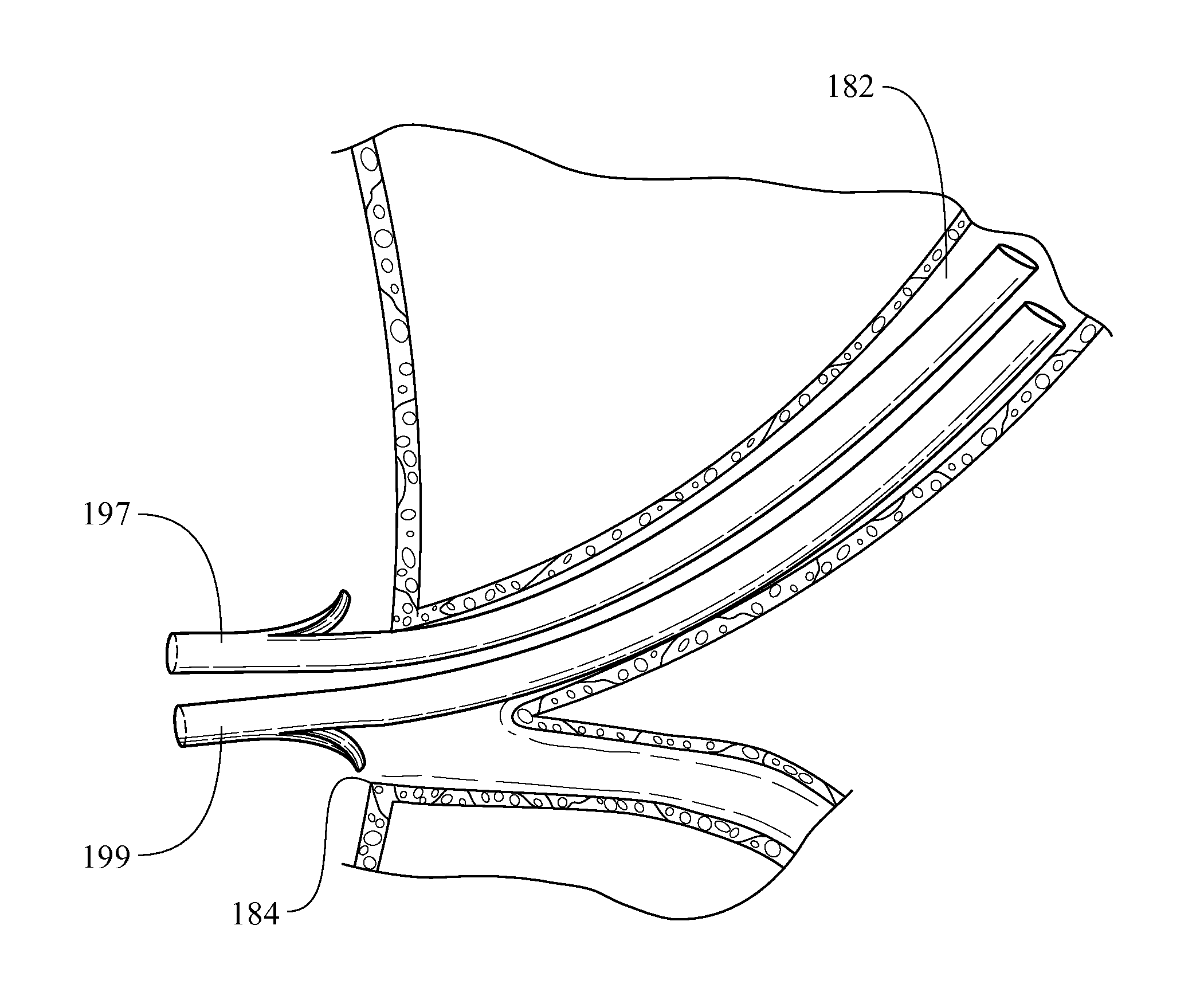
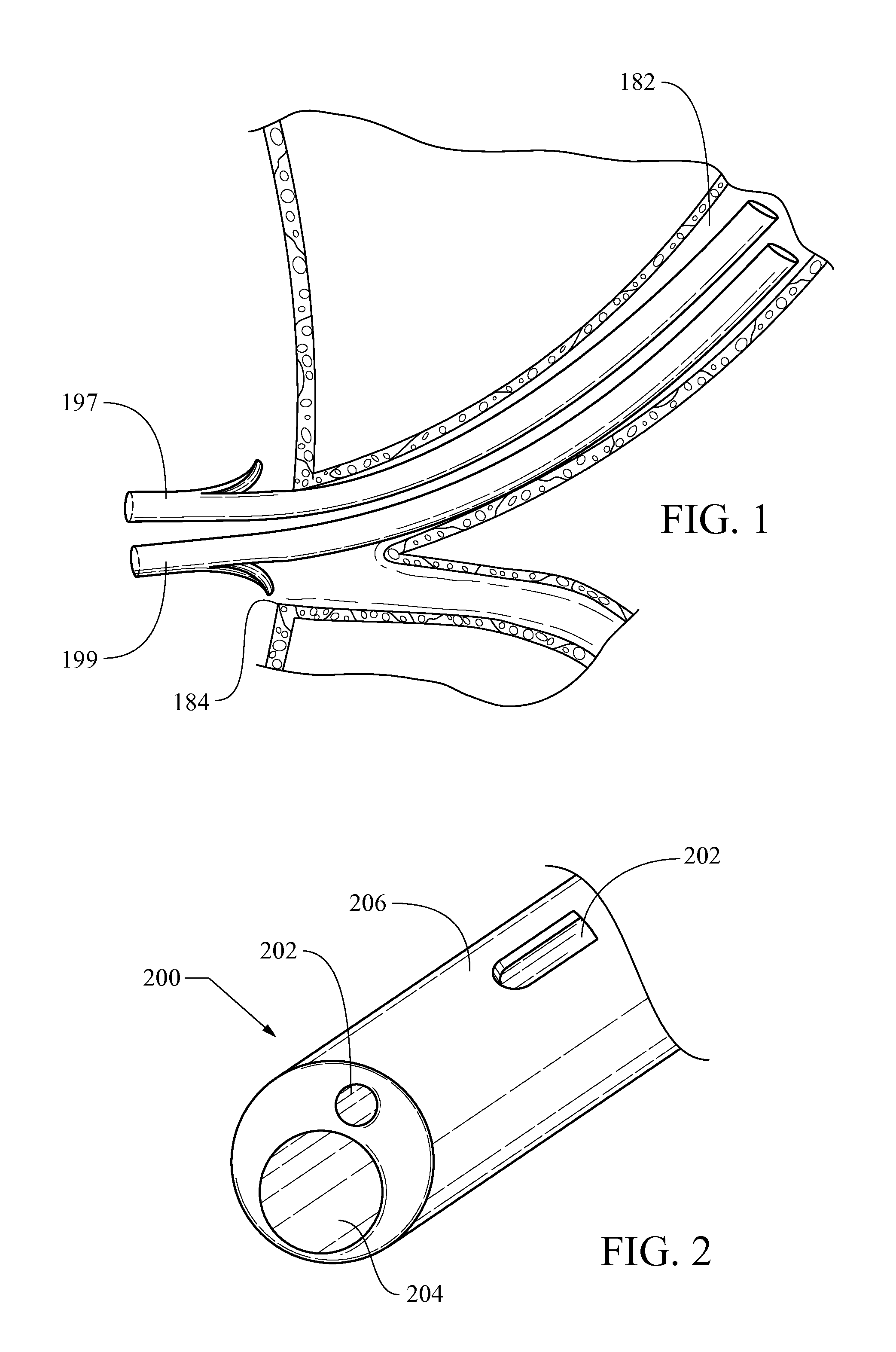
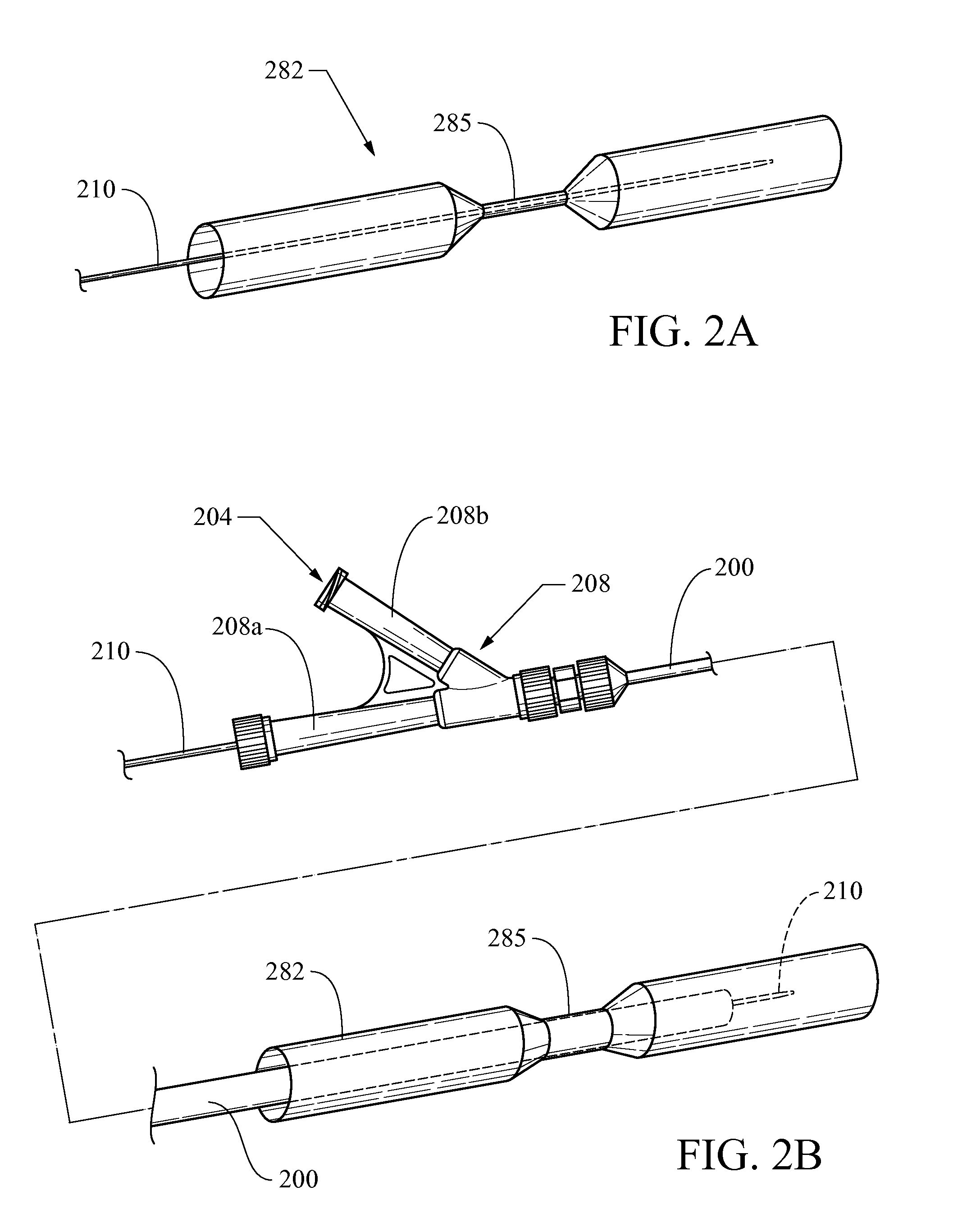
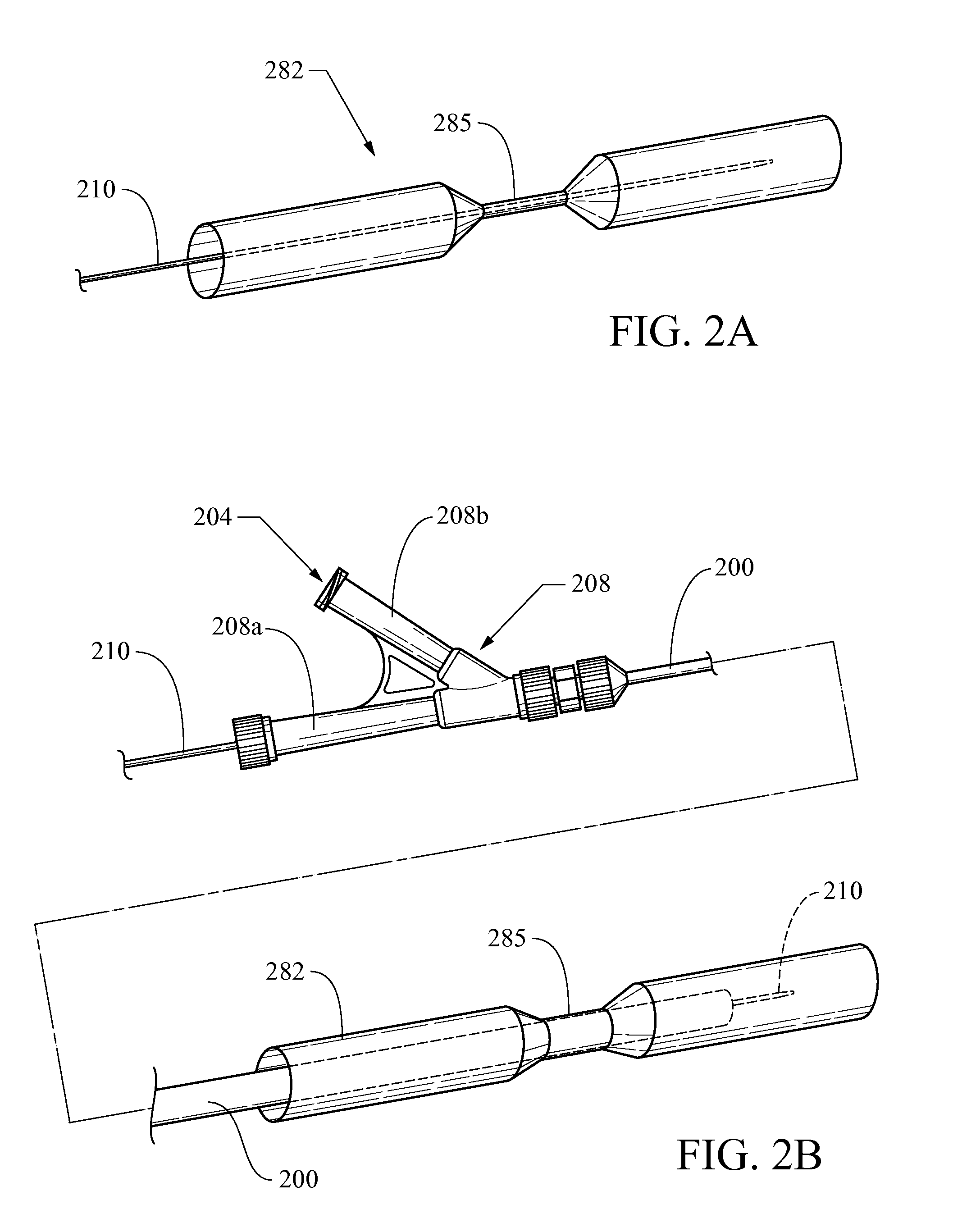
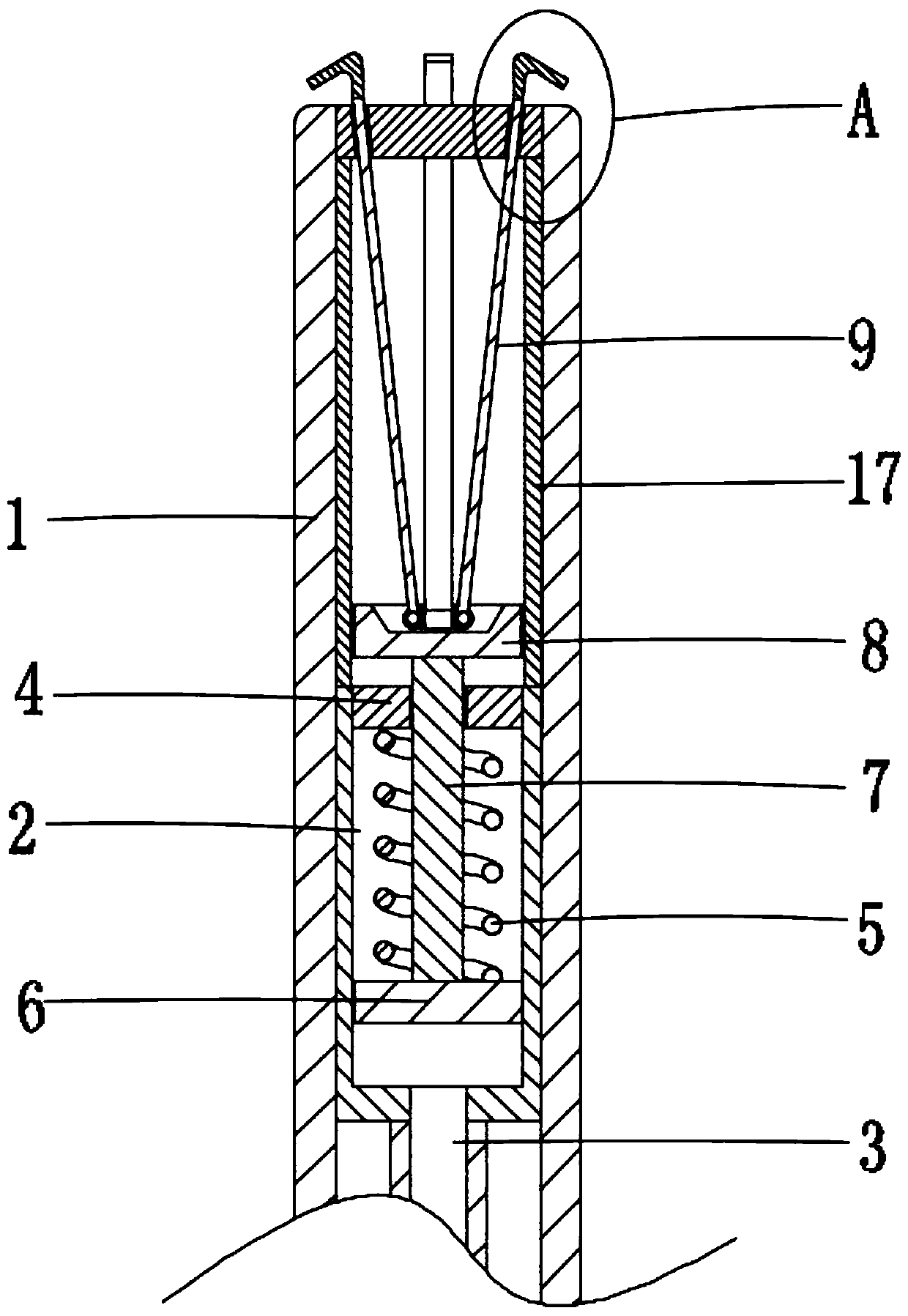
Biliary stent introducer system
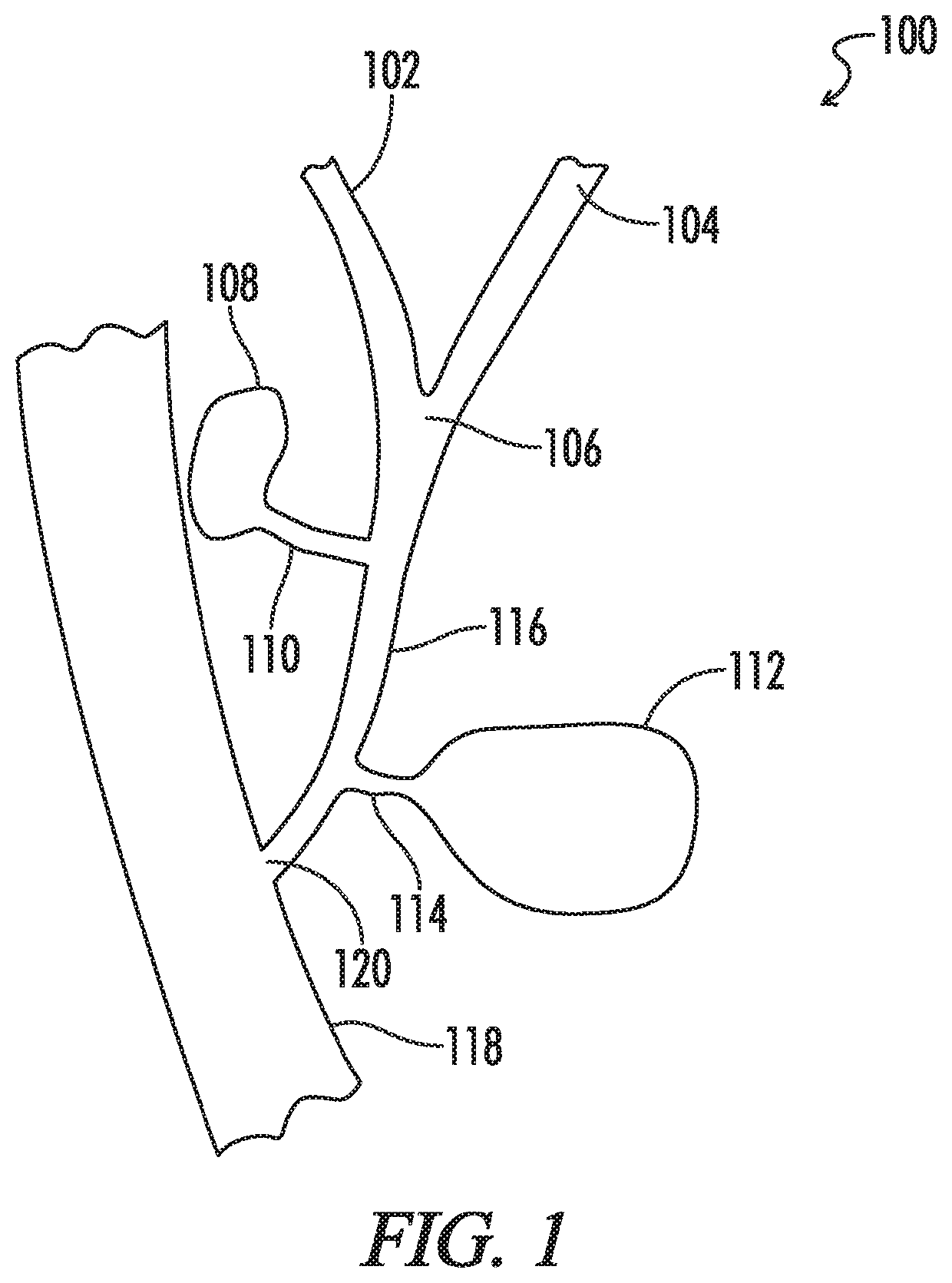
A stent delivery system and method for positioning a first and second stent in the main lumen and the first and second branch lumens of a bifurcation.
Owner:WILSONCOOK MEDICAL
Stent
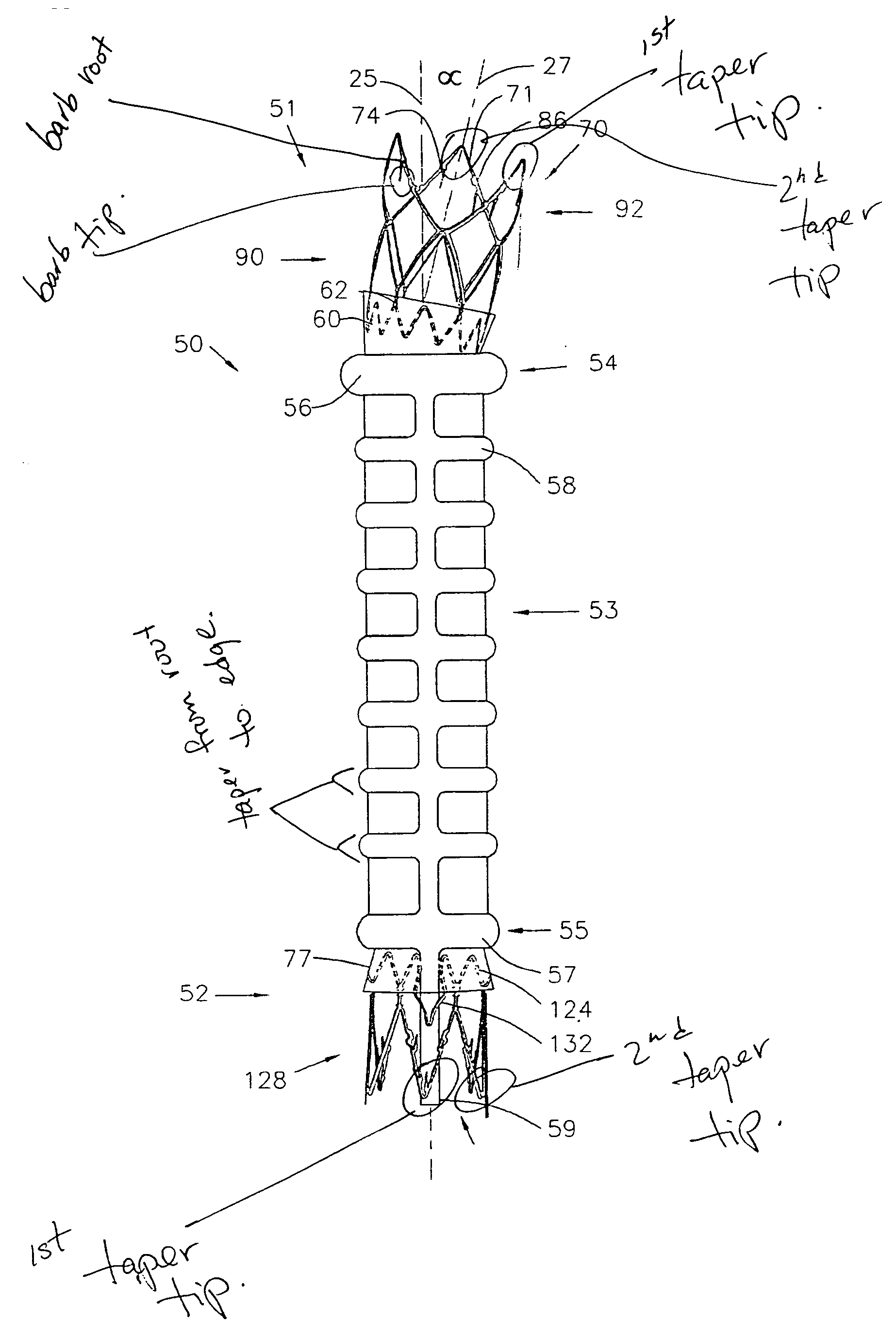
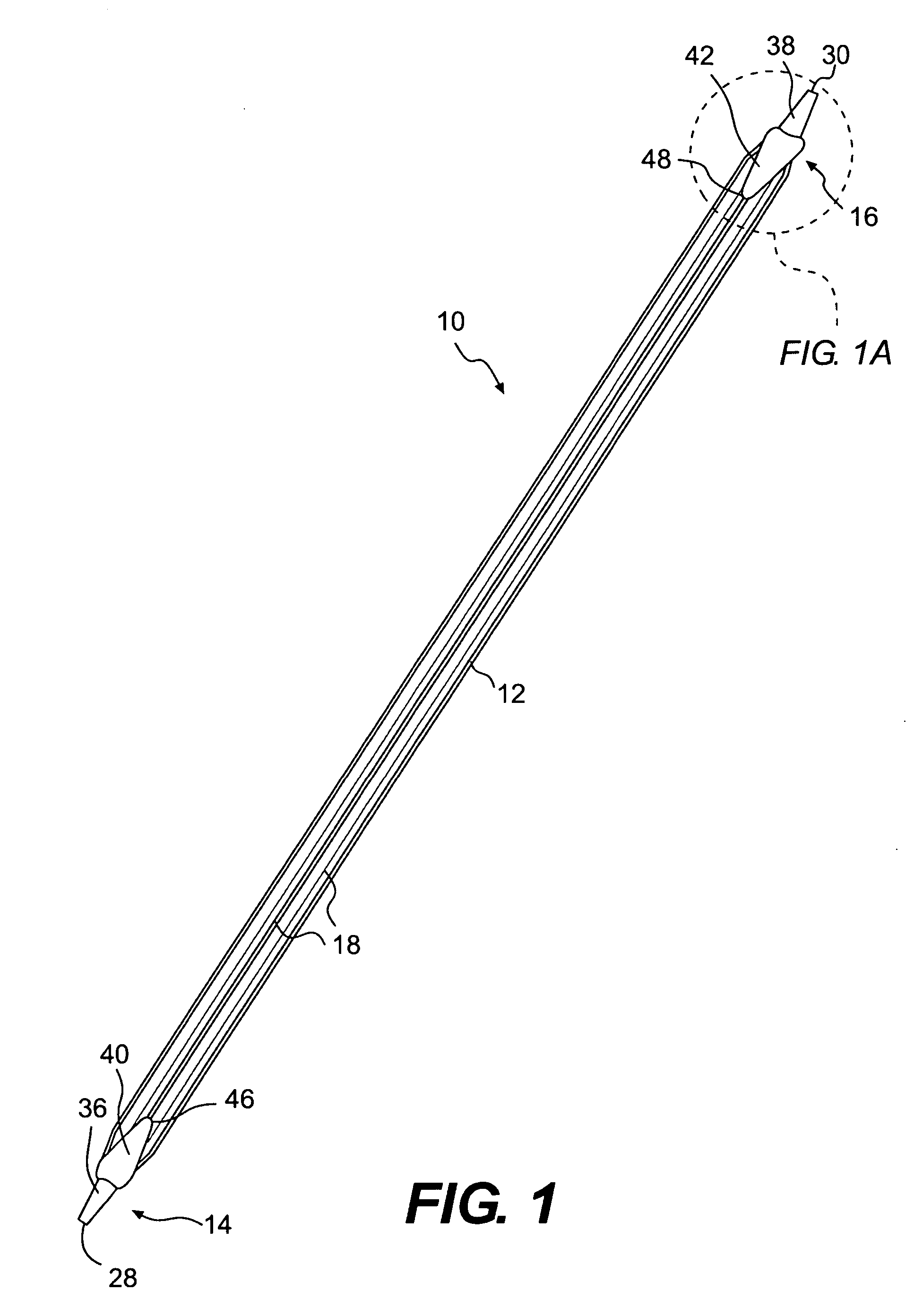
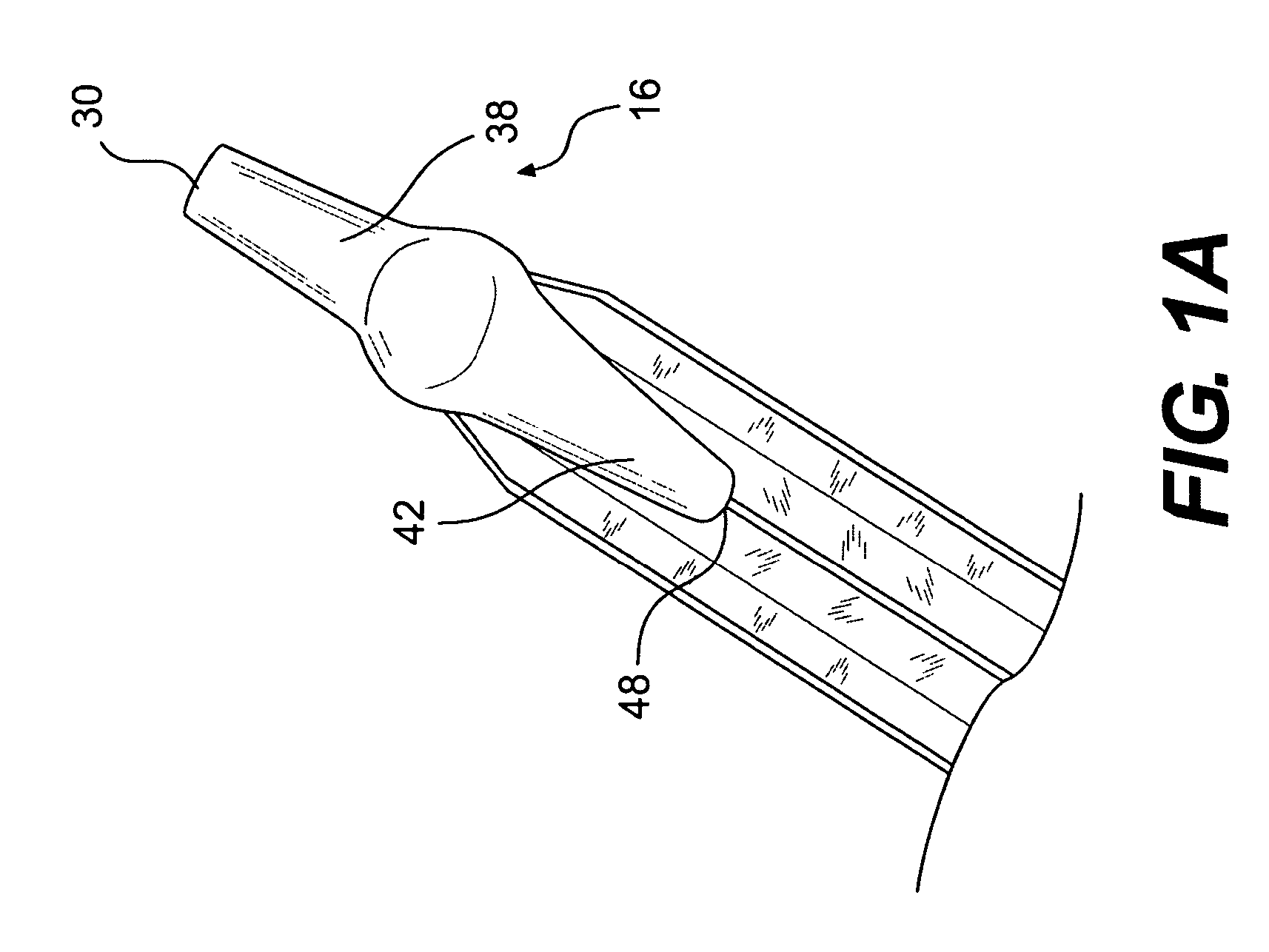
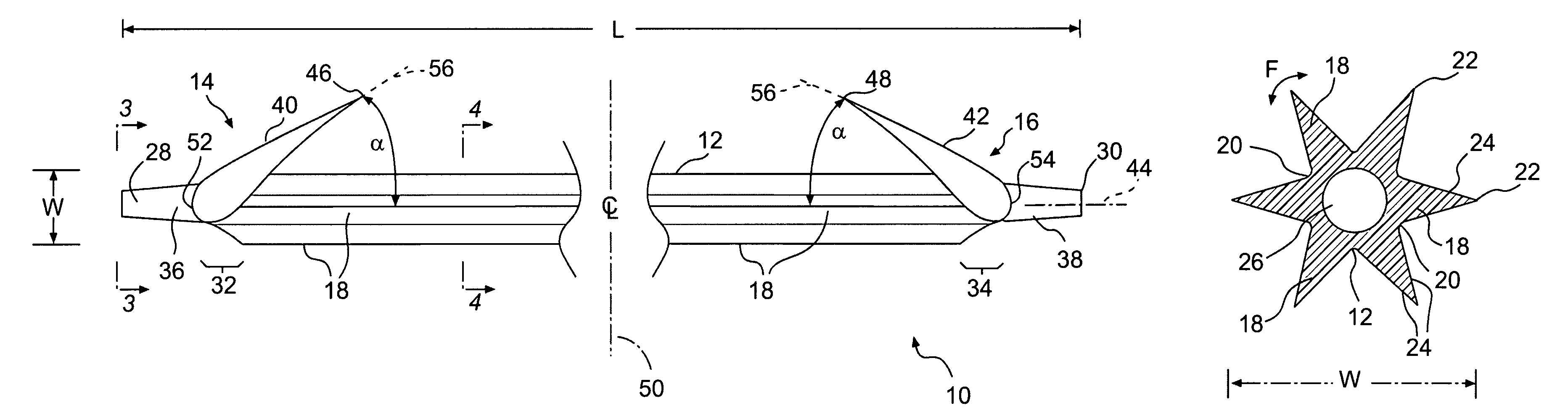
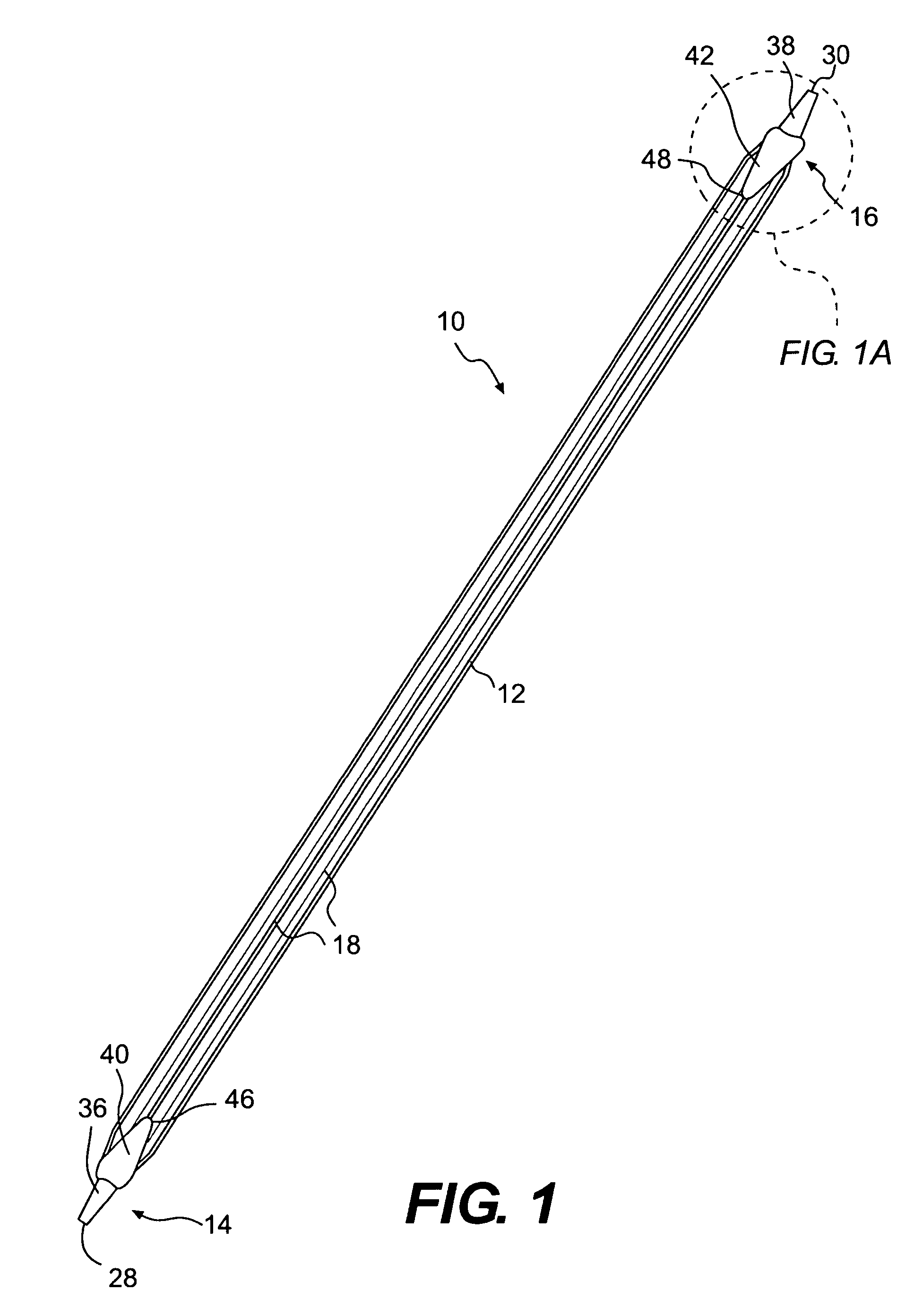
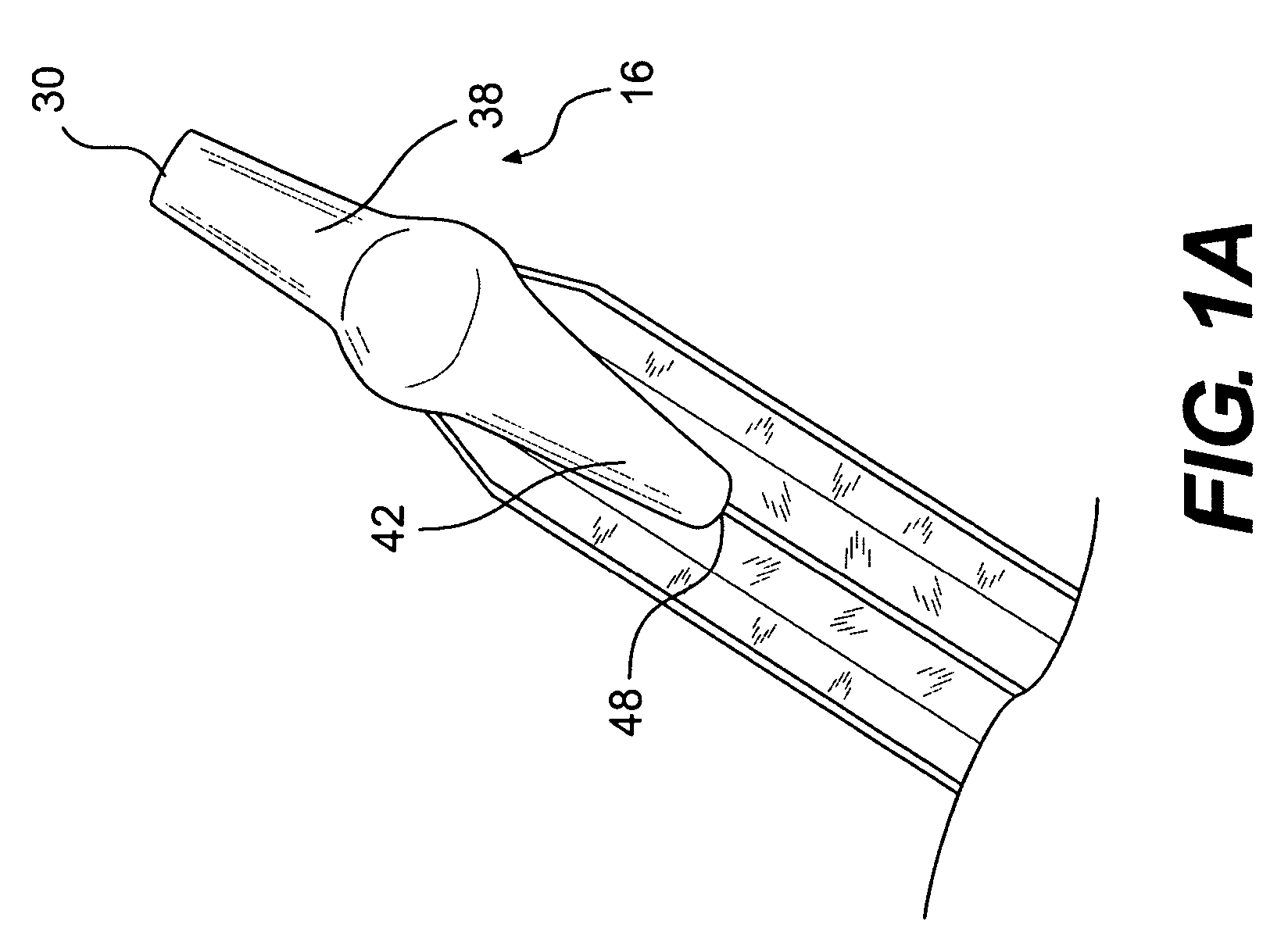
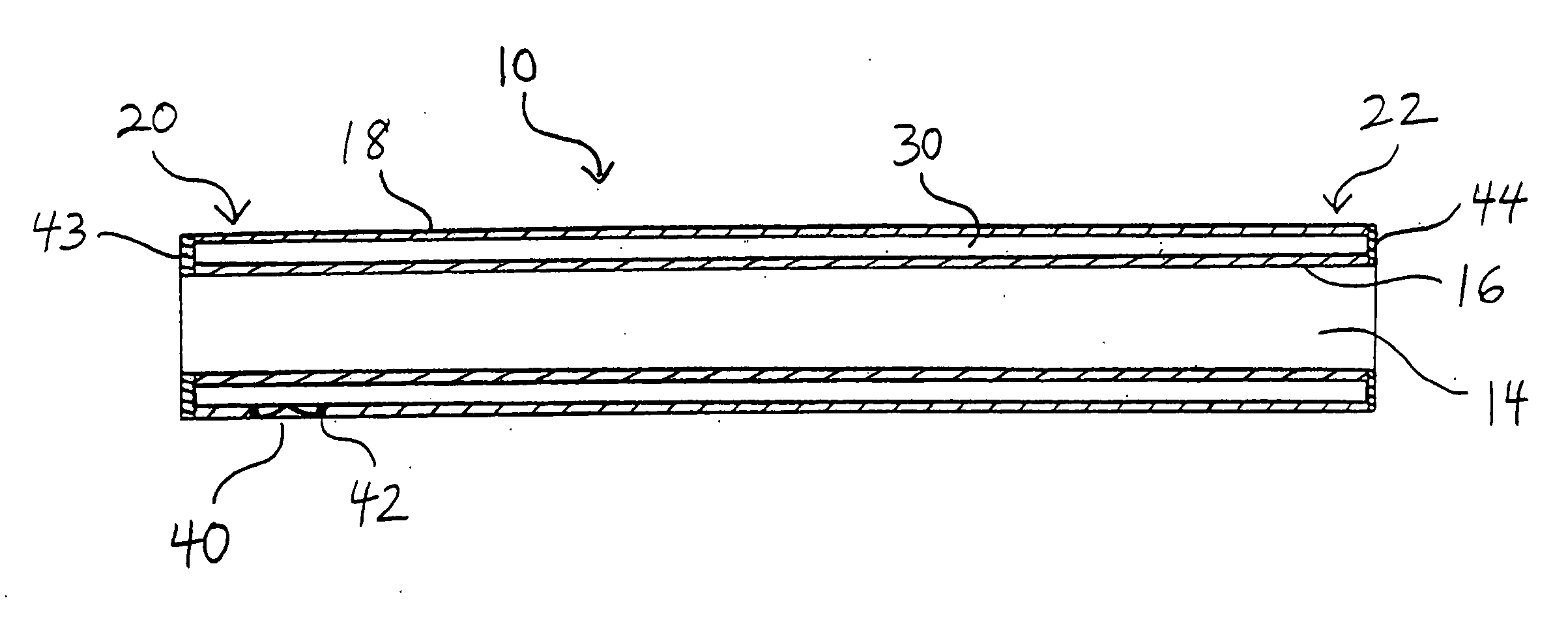
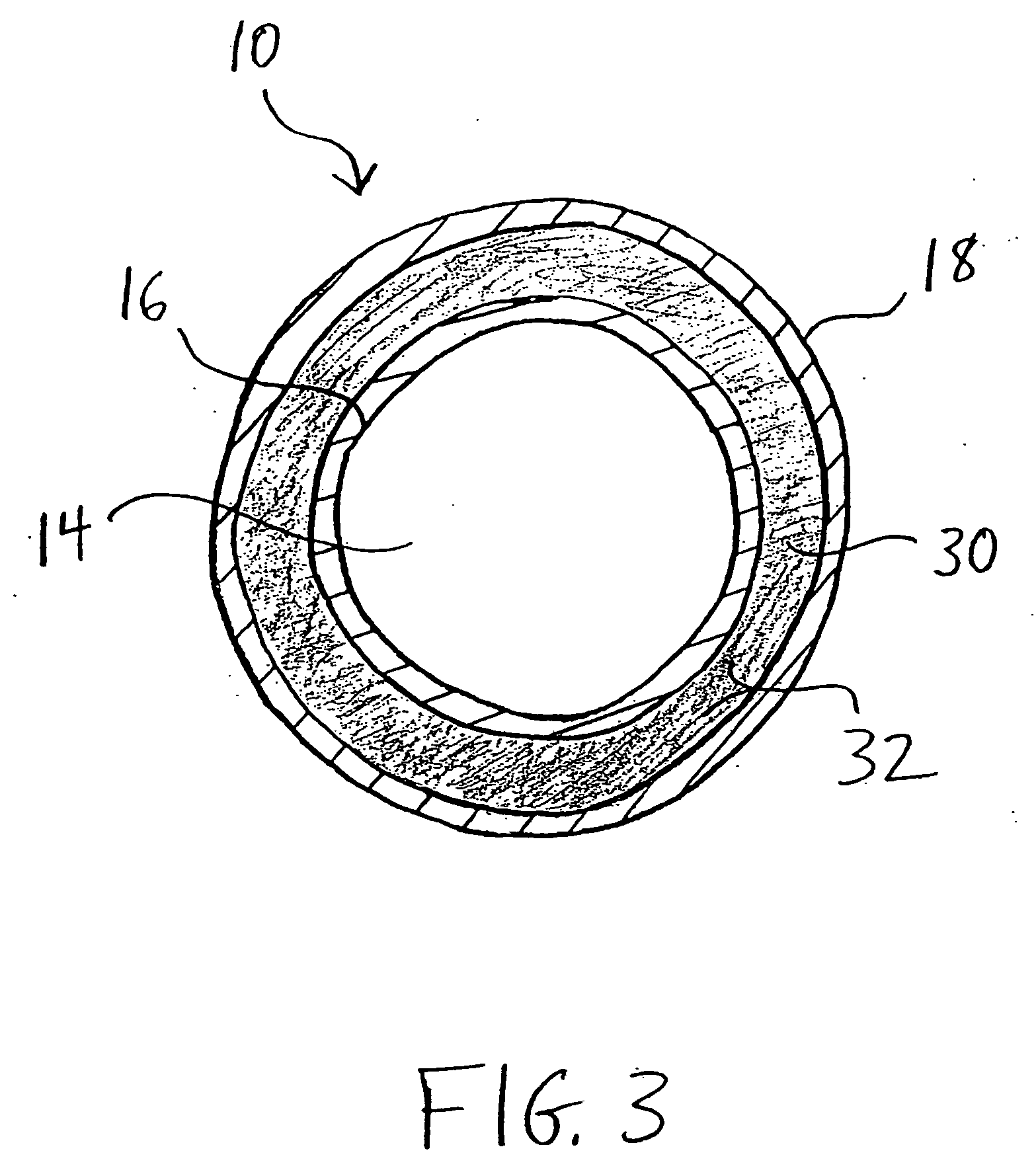
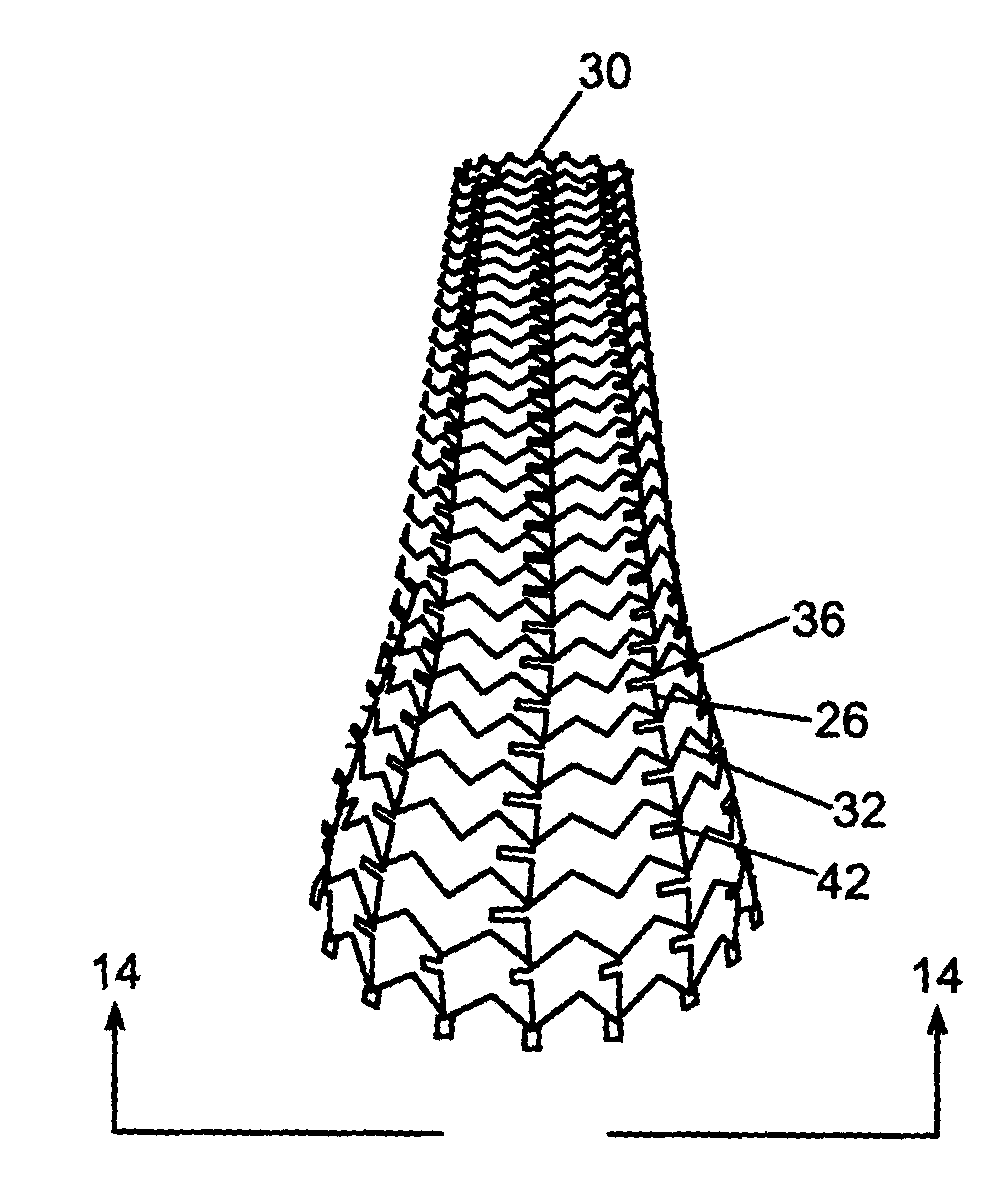
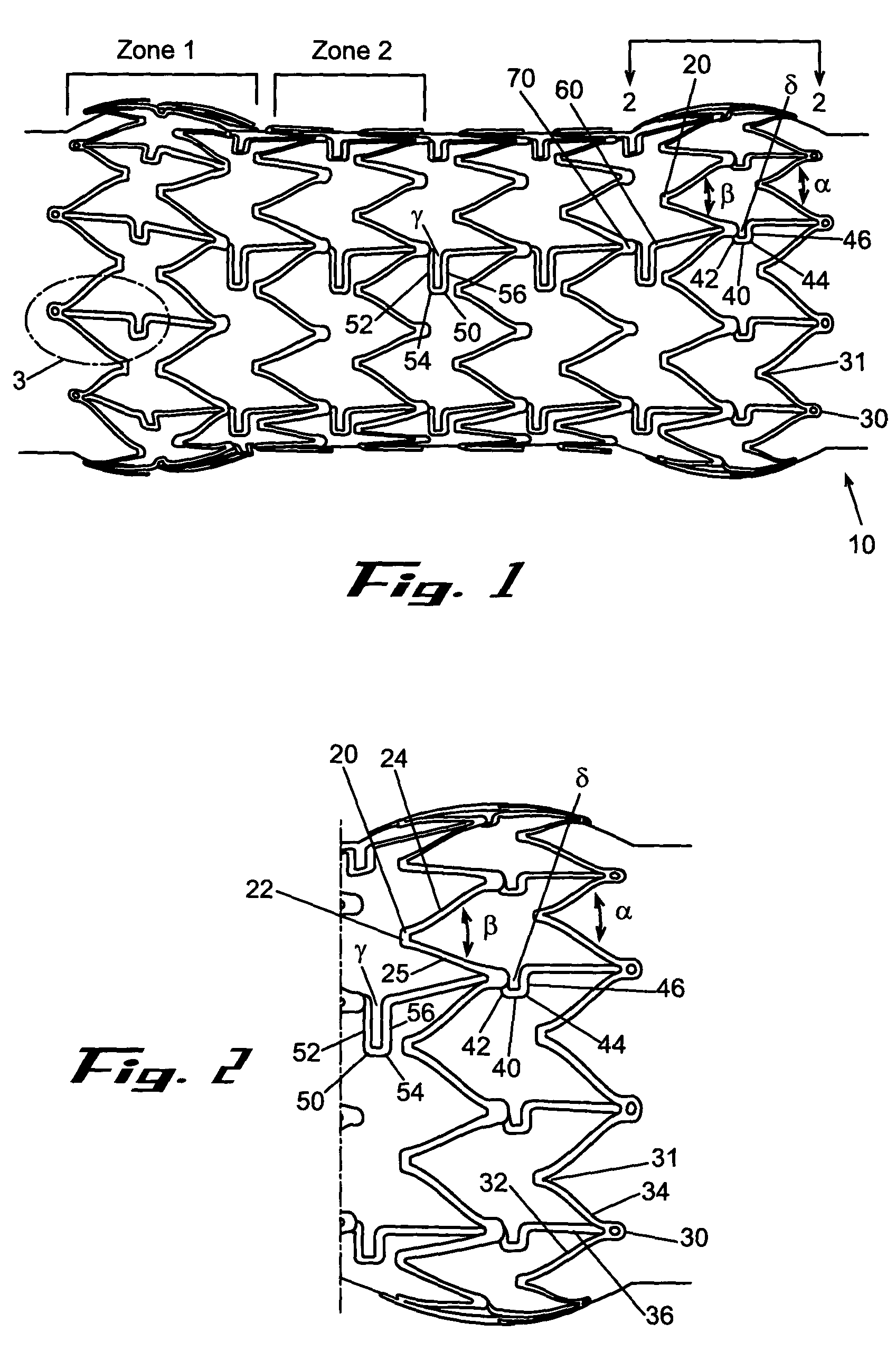
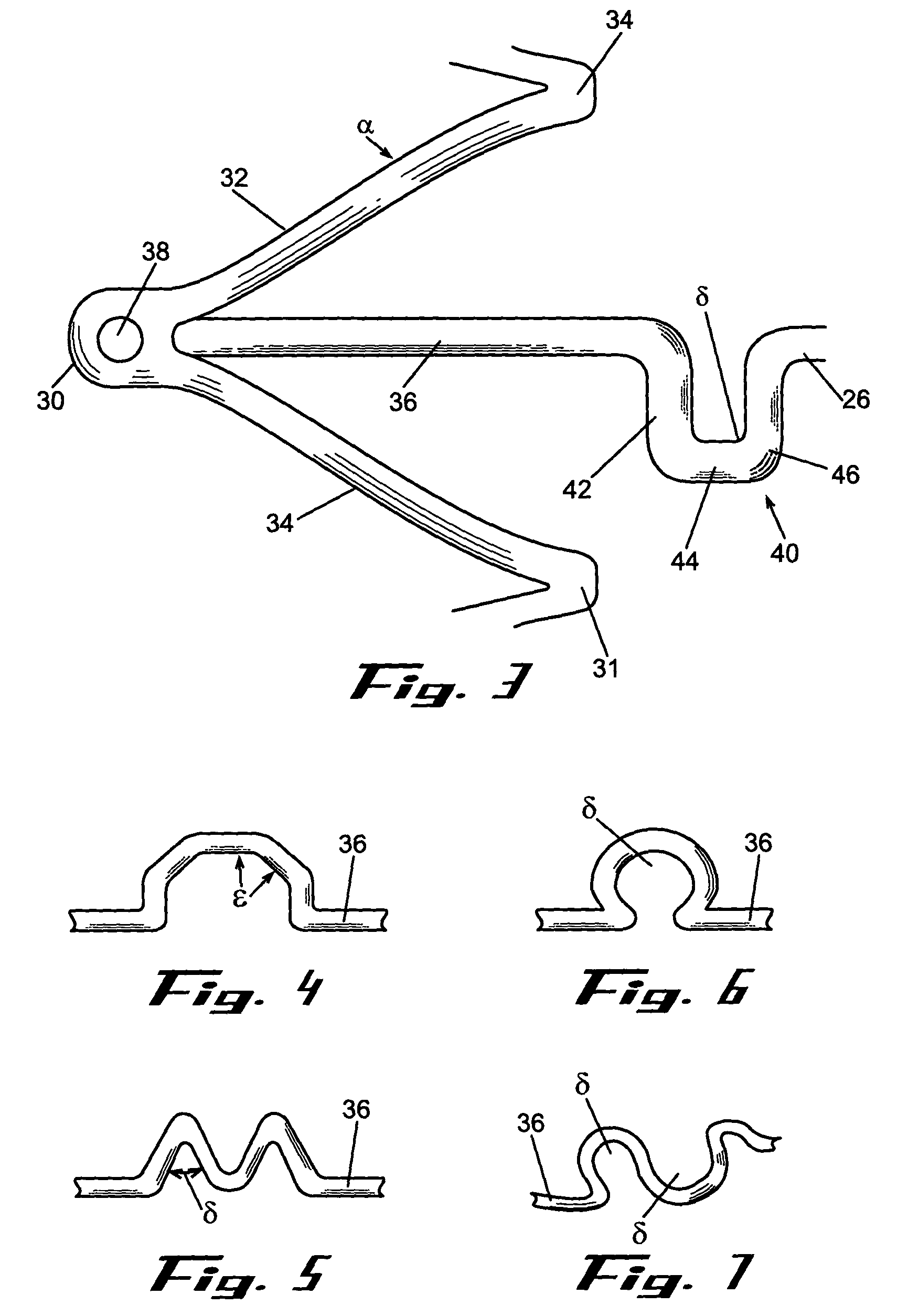
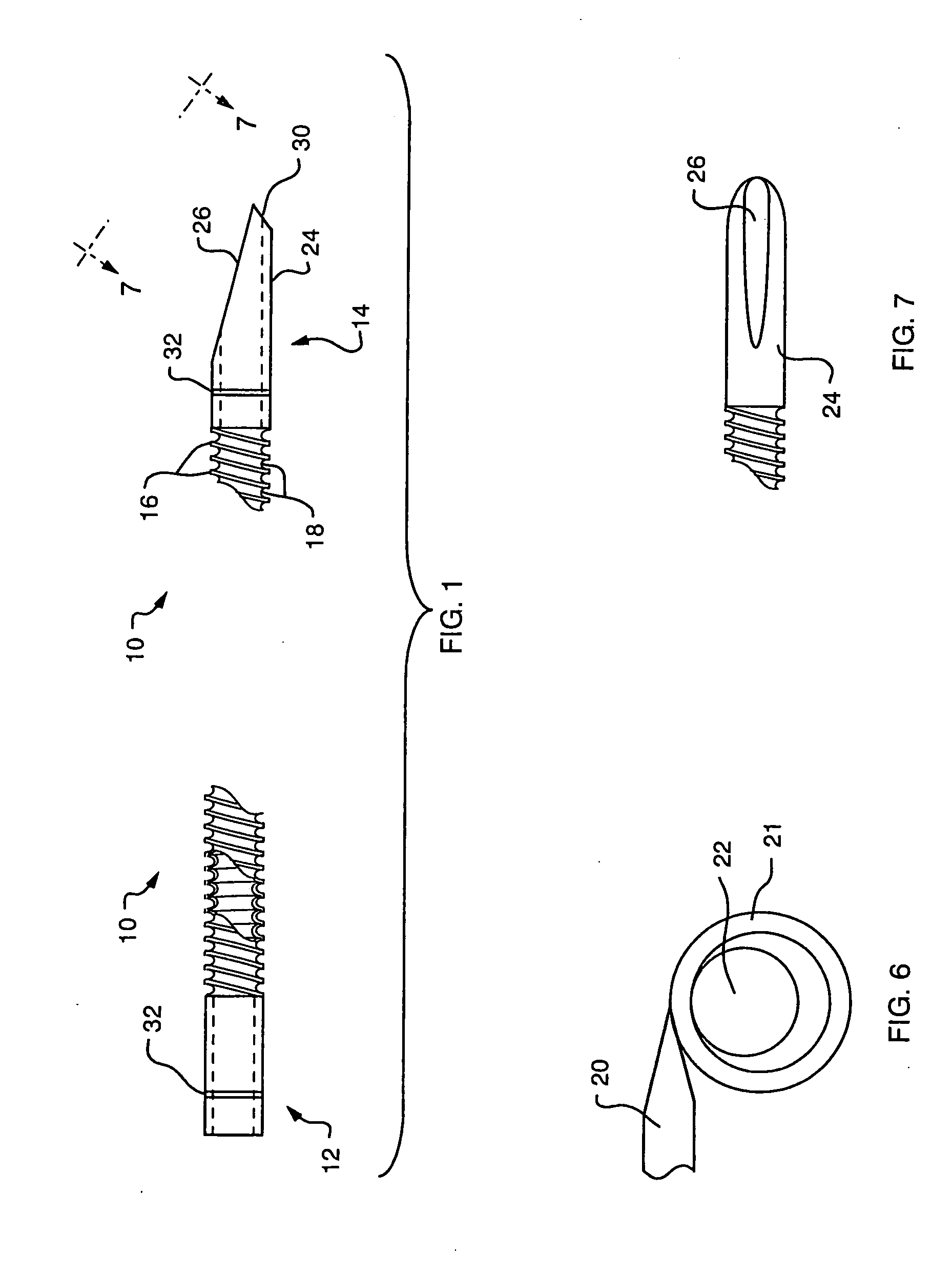
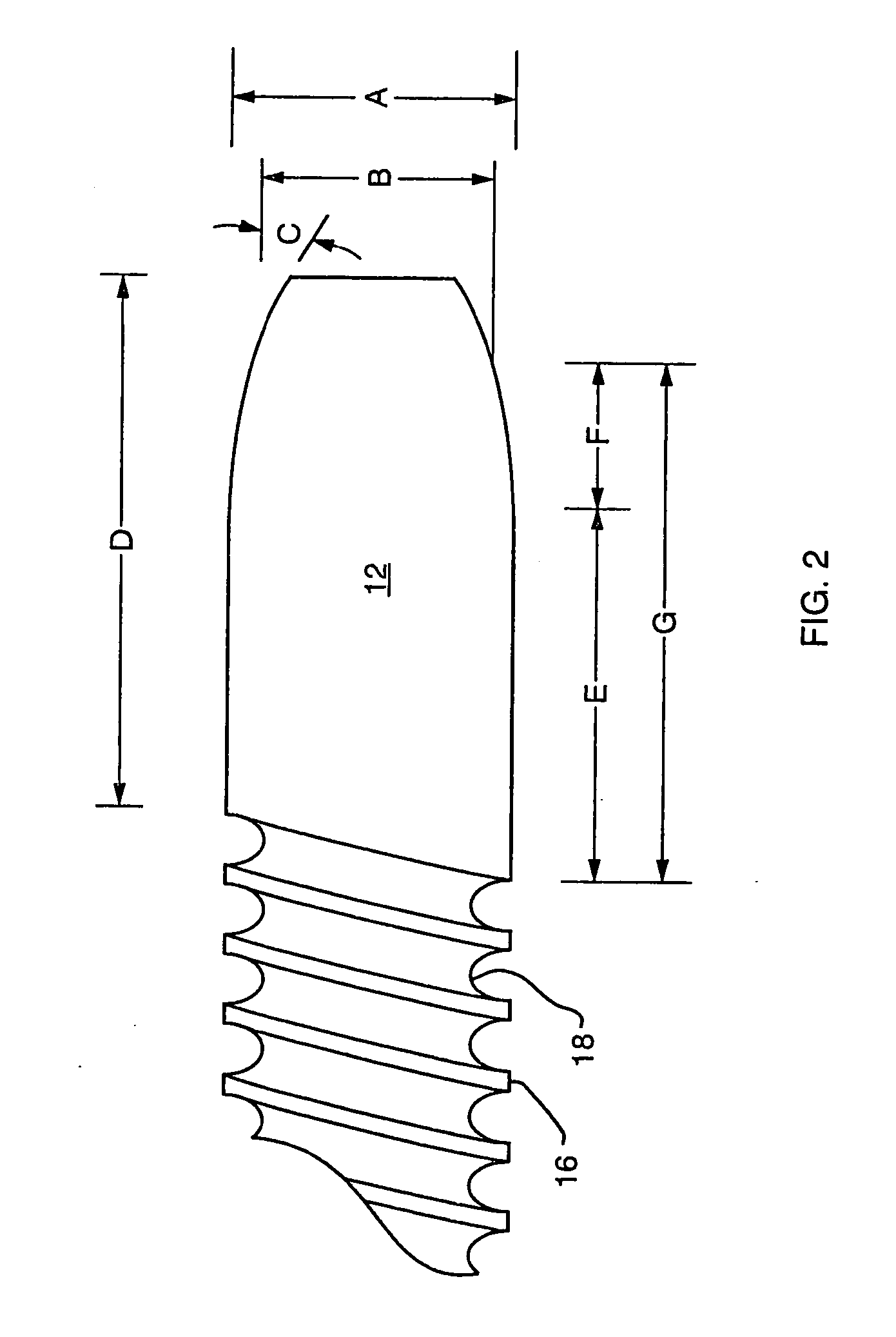
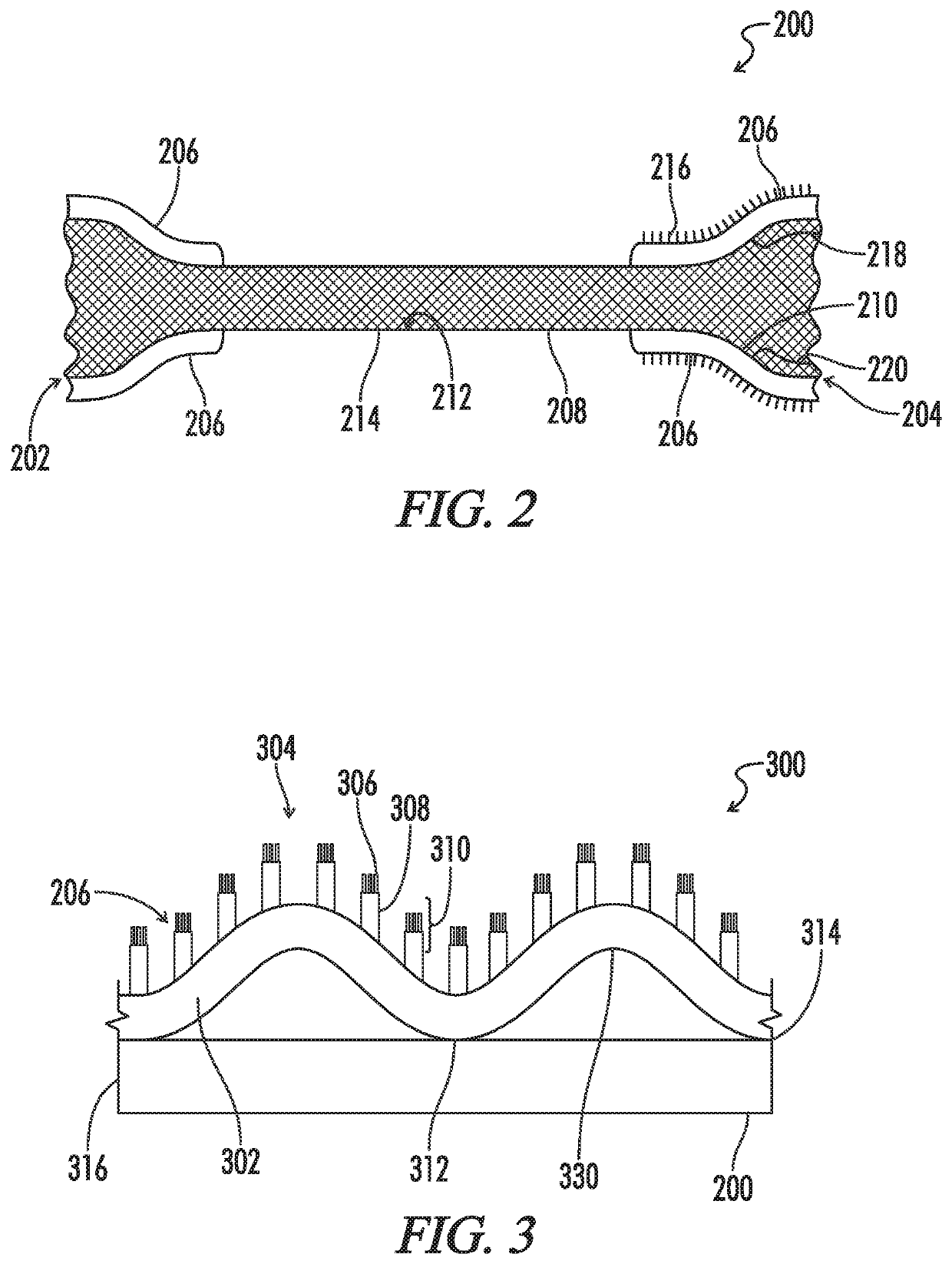
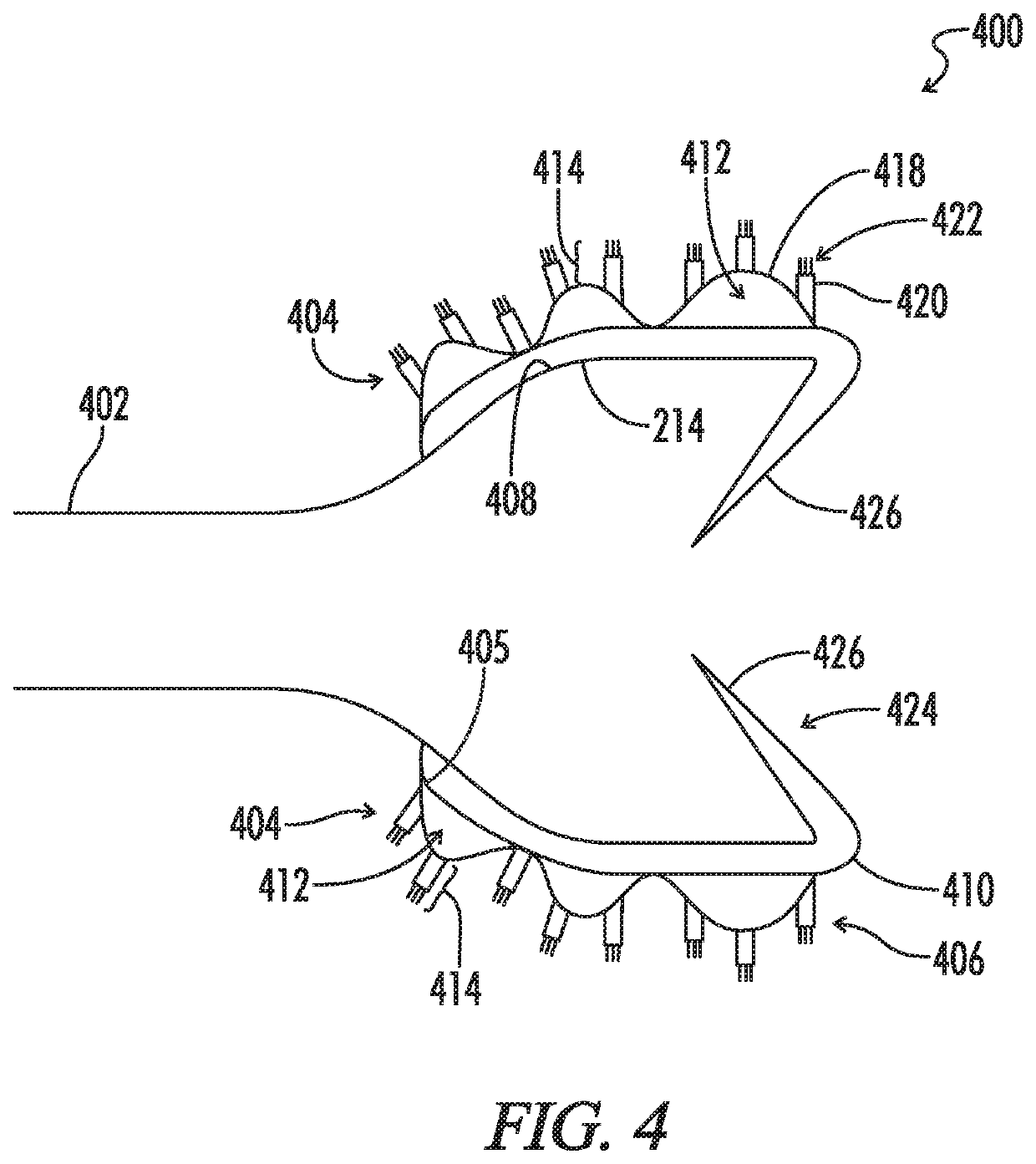
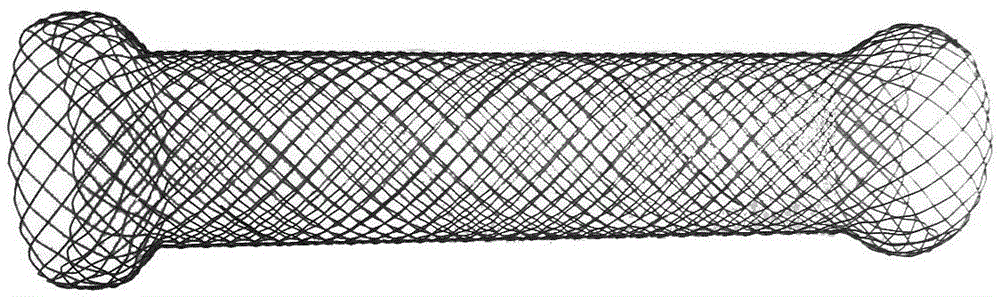
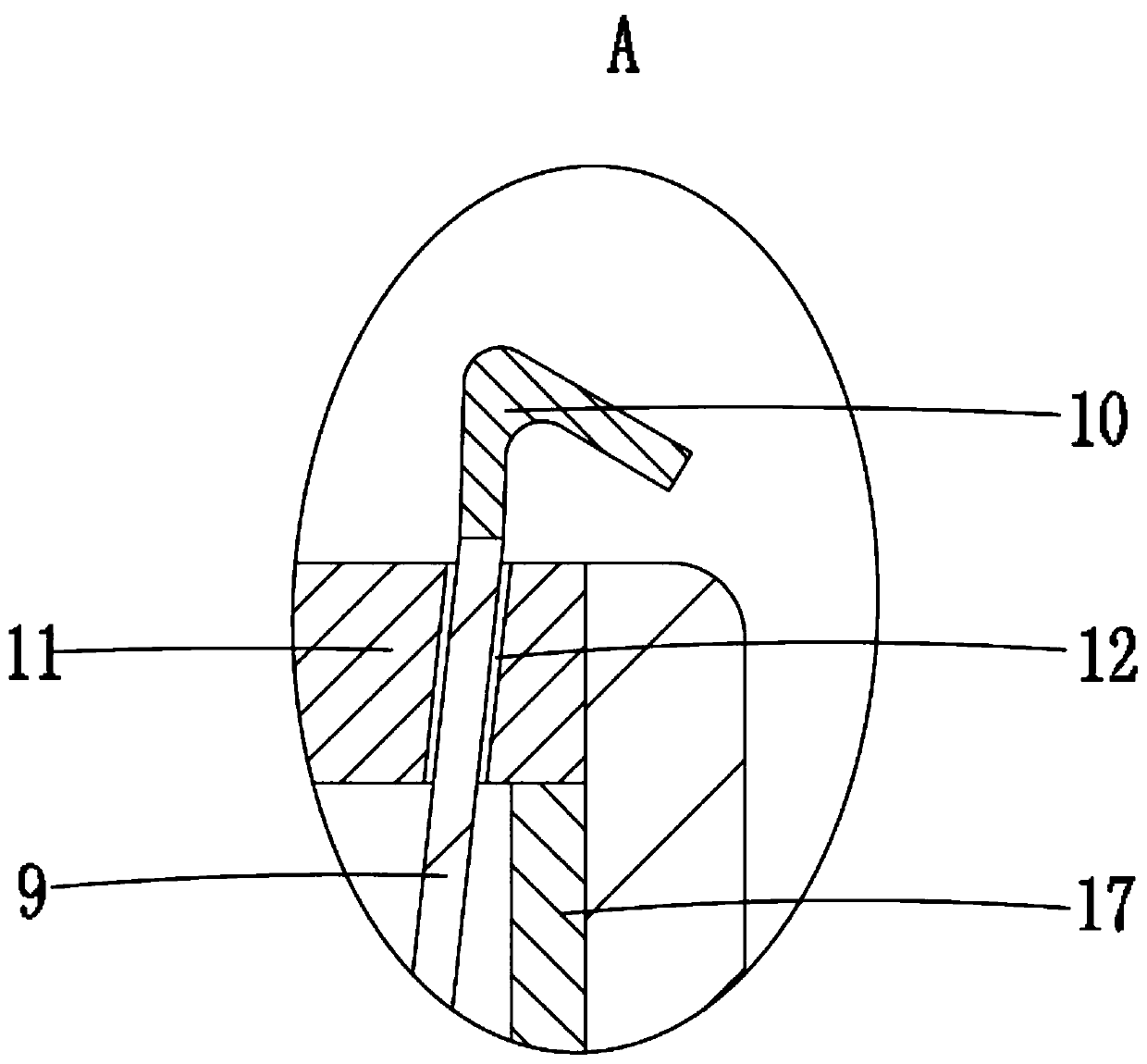
A biliary stent has a number of smooth-surfaced wings extending radially outwardly from a central region and extending longitudinally along substantially the entire length of the stent. A securement barb is located at one end of the stent and extends generally radially outwardly toward a side of the stent in cantilevered fashion at an angle less than or equal to 90°. The securement barb is angled such that it presents an outward-facing half-arrowhead profile. At least one end of the stent is smoothly or conically tapered. In one embodiment, the wings extend linearly along the stent; in another embodiment, the wings extend helically along the stent. Either embodiment may have a pigtail configuration at one or both ends.
Owner:CHEK MED SYST
Stent
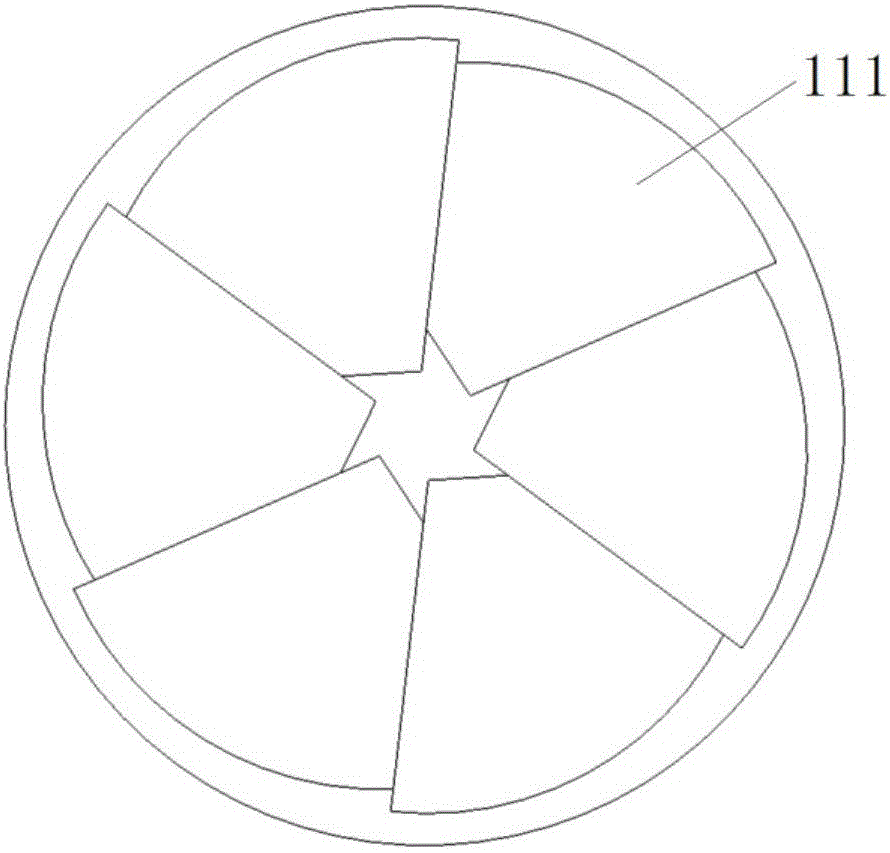
InactiveUS7338530B2Easy to manufactureFacilitate retention of stentStentsBile ductsEngineeringBiliary stent
A biliary stent has a number of smooth-surfaced wings extending radially outwardly from a central region and extending longitudinally along substantially the entire length of the stent. A securement barb is located at one end of the stent and extends generally radially outwardly toward a side of the stent in cantilevered fashion at an angle less than or equal to 90°. The securement barb is angled such that it presents an outward-facing half-arrowhead profile. At least one end of the stent is smoothly or conically tapered. In one embodiment, the wings extend linearly along the stent; in another embodiment, the wings extend helically along the stent. Either embodiment may have a pigtail configuration at one or both ends.
Owner:CHEK MED SYST
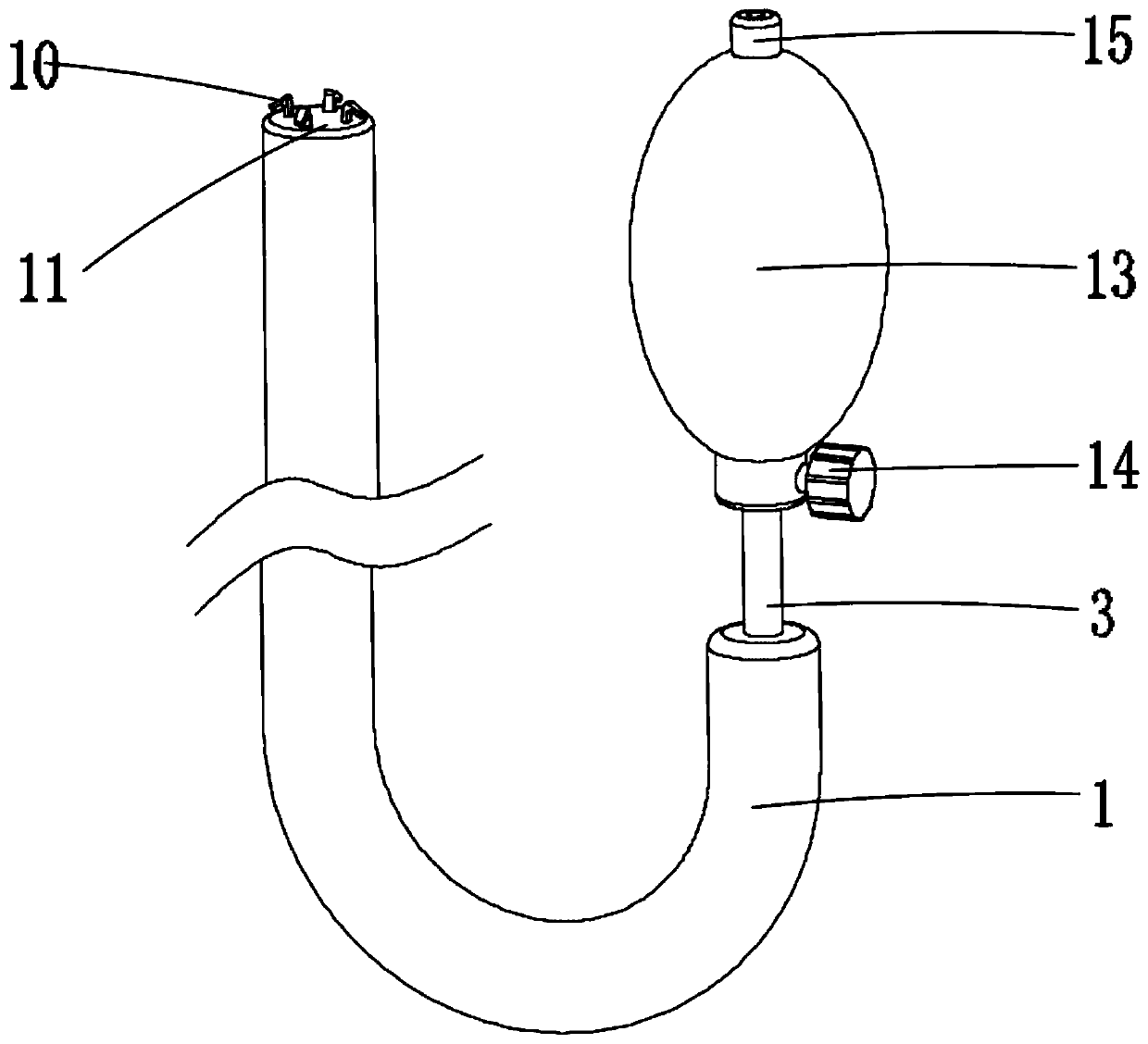
Inflatable biliary stent
An inflatable stent is provided for placement within the biliary duct to facilitate drainage therethrough and maintain the patency of the duct. The biliary stent includes a tubular member, an inflatable reservoir and a port located at a proximal portion of the tubular member. The tubular member has interior and exterior surfaces and an inflatable reservoir disposed circumferentially between the interior and exterior surfaces. An inflation fluid, such as a liquid or gas, may be inserted through the port and contained within the inflatable reservoir to expand the diameter of the biliary stent. The biliary stent may be removed from the patient by deflating the inflatable reservoir and using a removal device such as a forceps or snare.
Owner:WILSONCOOK MEDICAL
Removable biliary stent
The present invention, in an exemplary embodiment, provides a stent, which combines many of the excellent characteristics of both silicone and metal stents while eliminating the undesirable ones. In particular, a principal objective in accordance with the present invention is to provide a family of stents generally and a biliary stent in particular where the relative hardness / softness of regions of the stent can differ from other regions of the stent to provide additional patient comfort and resistance to radial forces. An exemplary embodiment also provides a family of stents with novel interstice configurations that facilitate flexibility, durability and / or proper installation. Additionally, the biliary stent in accordance with the present invention provides enhanced flow mechanics to ensure the adequate clearance of viscid fluids such as bile.
Owner:MERIT MEDICAL SYST INC
Polylactic acid degradable film
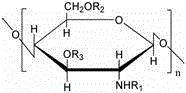
The invention relates to a polylactic acid degradable film with controllable degradable time, belonging to the technical field of medical appliances. The polylactic acid degradable film comprises the following components in percentage by weight: 1%-35% of plasticizer and 65%-99% of polylactic acid, polylactic acid-glycollic acid copolymer, polyglycollic acid-epsilon-caprolactone copolymer, polylactic acid-epsilon-caprolactone copolymer or polylactic acid-glycollic acid-epsilon-caprolactone copolymer, wherein the plasticizer is more than one of citrate ether, glucose monoester ether, fatty acid ester ether, polyethylene glycol 400-2,000, low-molecular weight polylactic acid, glycerol, glycerol triacetate and ethylene glycol monobutyl ether acetate. The manufactured polylactic acid degradable film of the invention has good strength and controllable degradable time, can be used for a human body, such as an anti-adhesion film in a surgical operation, a digestive stent, a biliary stent, a prostate stent, an endovascular stent, an intrauterine stent and a medical appliance fitting, and can be used for preparing the required controllable polylactic acid degradable film according to the clinical requirements.
Owner:山东省医疗器械研究所
Stent delivery catheter
A delivery device for a biliary stent constructed of thermoplastic material with ridges and valleys formed along the outer surface of the stent that provide a helical, thread-like configuration. The delivery device comprises an external surface that is selectively expandable to engage an interior surface of the tubular stent. Additionally the exterior surface may include ridges and valleys that coincide with ridges and valleys defined by the helical thread extending through the stent in order to provide a more secure engagement with the stent during delivery.
Owner:CR BARD INC
Medicine-carrying nanofiber membrane biliary stent and preparation method thereof
InactiveCN103655015APlay a sustained release roleIncreased degradation rateStentsSurgeryMicro nanoFiber
The invention discloses a medicine-carrying nanofiber membrane biliary stent and a preparation method thereof. The medicine-carrying nanofiber membrane biliary stent comprises one part of nanofiber inner layers, the other part of nanofiber inner layers, a metal support and a medicine-carrying degraded fiber outer layer from inside to outside, wherein one part of nanofiber inner layers are arrayed in the pipe diameter direction, and the other part of nanofiber inner layers are arrayed disorderly. The nanofiber inner layers arrayed in the pipe diameter direction and the nanofiber inner layers arrayed disorderly are bonded together. The nanofiber inner layers arrayed disorderly and the medicine-carrying degraded fiber outer layer are boned together through meshes of the metal support, and the metal support is firmly fixed in a fiber membrane. The preparation method includes that electrostatic spinning equipment is adopted for electrostatic spinning. Compared with a micro-nano ball, the nanofiber membrane biliary stent has better structural stability, can be used for treating biliary stricture caused by tumors, has a mechanical expansion effect and a local treatment function, and can solve the biliary malignant stricture better than a common stent or a membrane-covering stent. In addition, the method can be applied to gastrointestinal tract stents and tracheal stents.
Owner:张建平
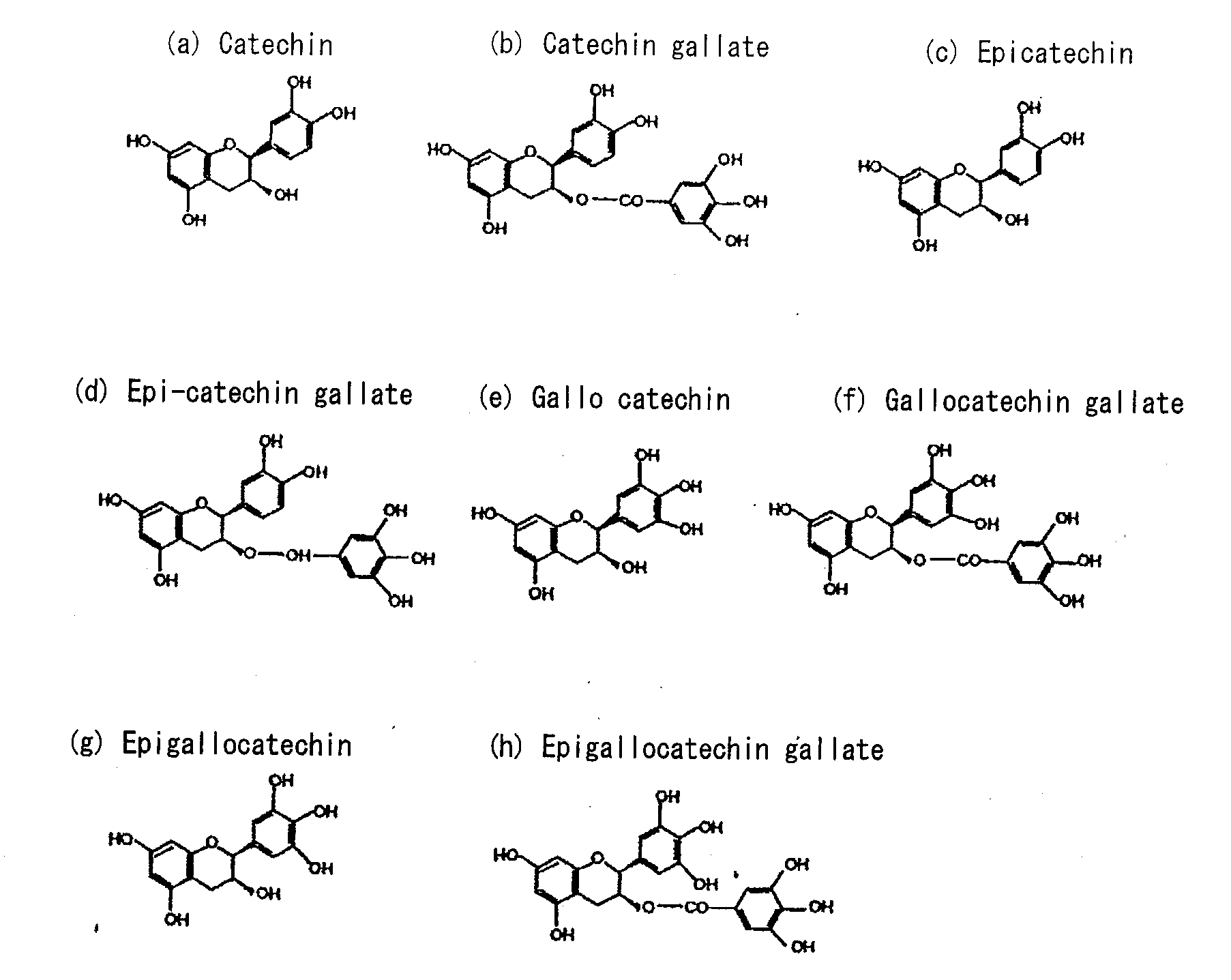
Biofilm formation inhibitor and treatment device thereof
InactiveUS20090099646A1Effectively and continuously inhibiting biofilm formationPrevent bacterial growthAntibacterial agentsBiocideBiological bodyBiofilm
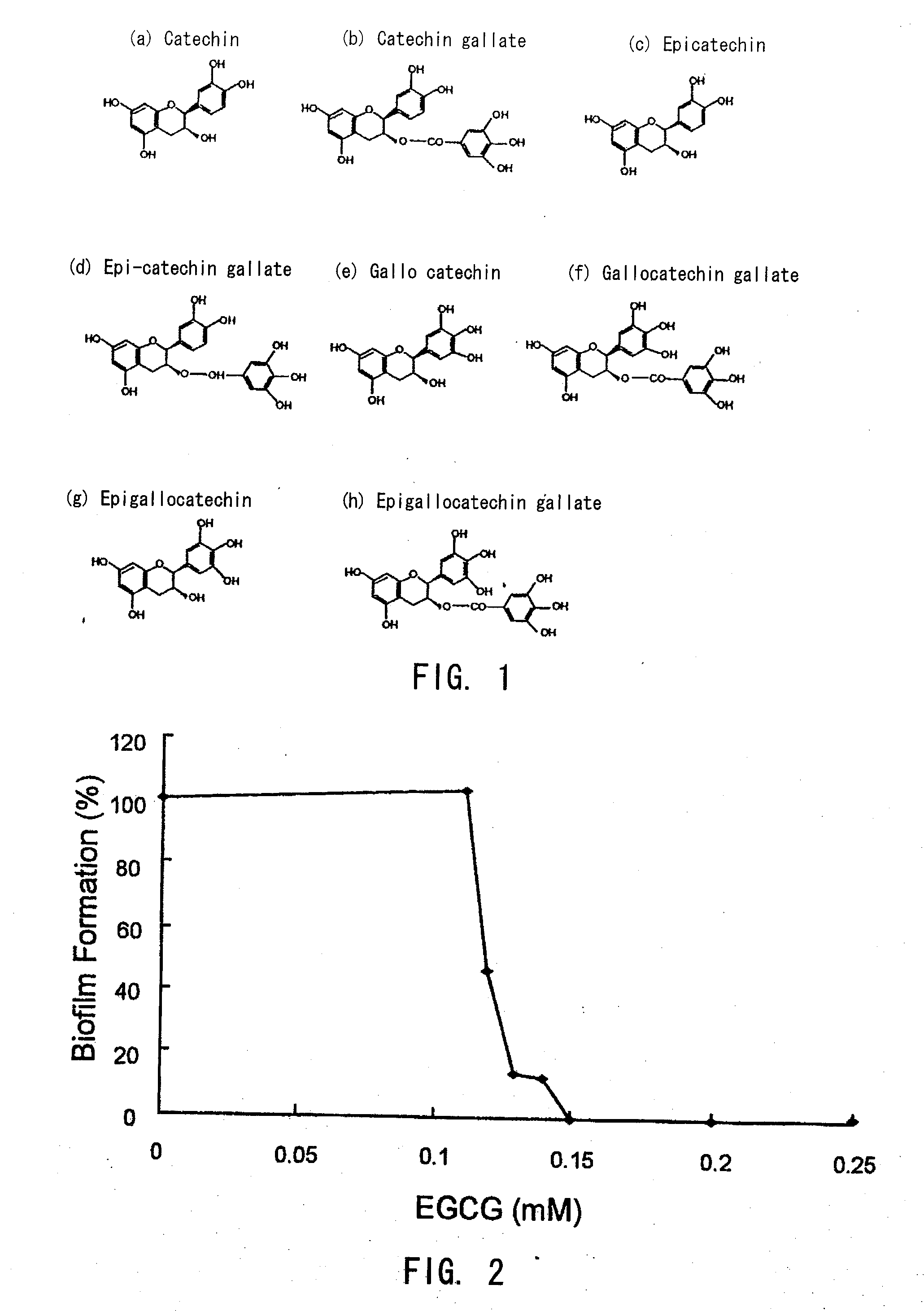
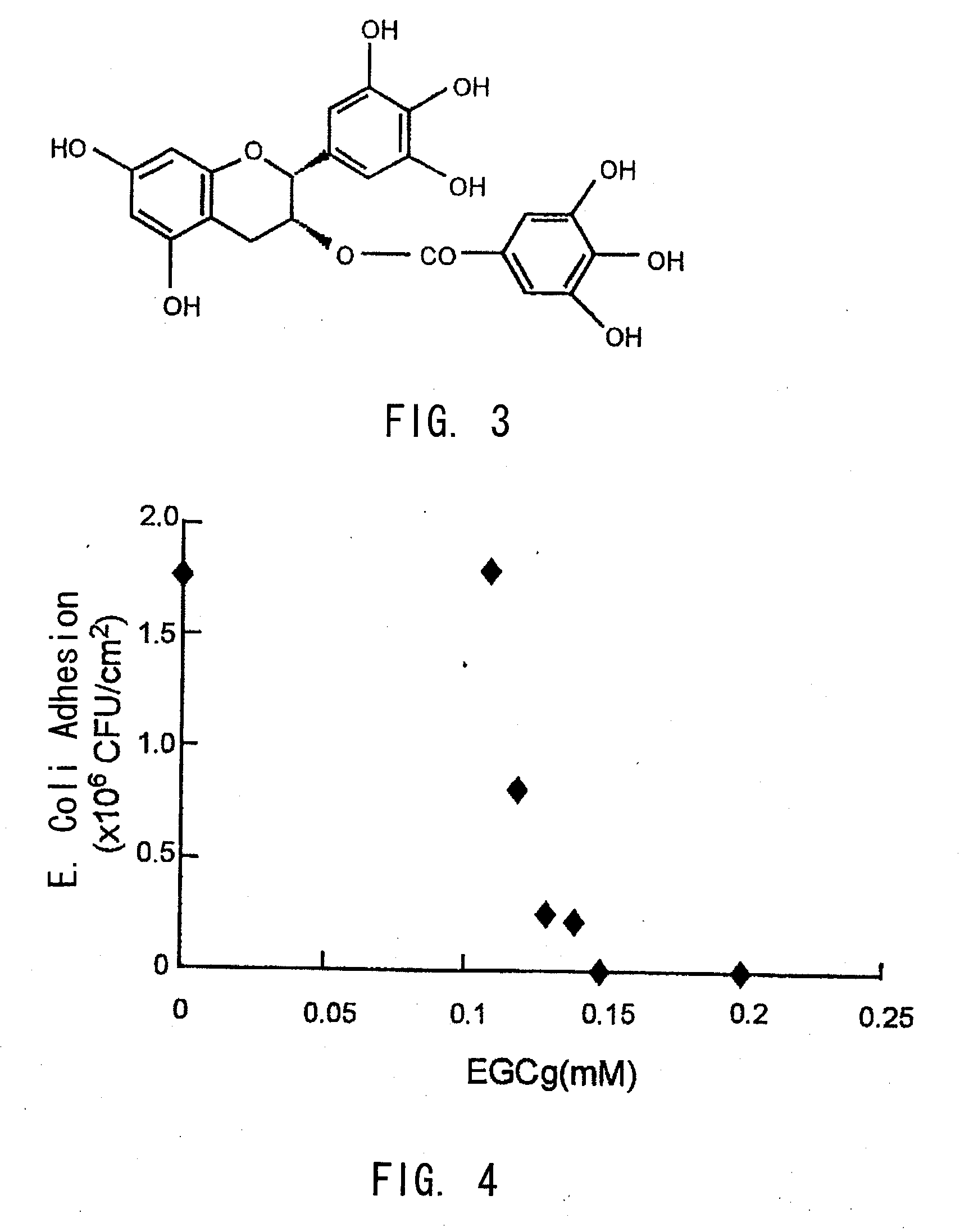
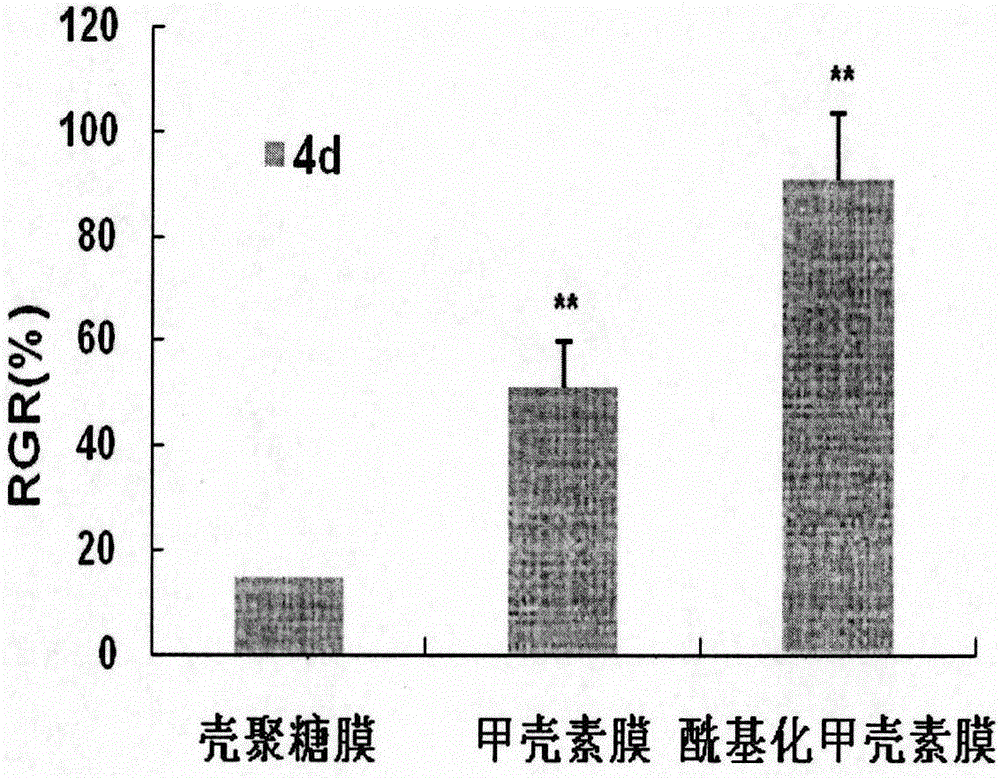
A treatment device such as a biliary stent, capable of effectively and continuously inhibiting biofilm formation, and a biofilm formation inhibitor suitable for coating on the treatment device by killing all bacteria including E. coli and by suppressing the growth of these bacteria, are provided. A biofilm formation inhibitor having an effective amount of catechin for inhibiting biofilm formation and a carrier that is suitable for adhesion of said catechin to a treatment device to be placed in the living body, are provided.
Owner:KYUSHU UNIV
Lumen stent capable of conducting development absorption and preparation method and application thereof
ActiveCN105148330AHigh mechanical strengthGood grip rebound performanceSurgeryCoatingsUrethraInsertion stent
The invention discloses a lumen stent capable of conducting development absorption. The lumen stent is characterized in that the lumen stent is of a hollow tubular structure and made ofacyl amino polysaccharide, the outer surface of the tubular structure is provided with a development coating, and the tube wall is provided with or not provided with a transparent hole structure or a pattern structure. The lumen stent can be used as a blood vessel stent, a biliary stent or a urethral stent and is used for treating narrowing of the blood vessel, the bile duct and the urine tube cavity, capable of conducting development, capable of being degraded and absorbed in a human body, the situation that the lumen stent stays in the body for a long time can be avoided, and the lumen stent has wide market prospect.
Owner:青岛健康海洋生物制药有限公司
Biliary Stent
ActiveUS20200030124A1Maintaining luminal patencyPromote migrationStentsBile ductsBiliary stentBiomedical engineering
The present disclosure provides an endoprosthesis where a preferably polymeric coating has a number of surface features such as protrusions or textures that are arranged in a micropattern. The endoprosthesis optionally has an expanded state and a contracted state, and in some cases includes a stent with a polymeric coating attached to an outer surface of the stent. The stent may have an inner surface defining a lumen, an outer surface, and a stent thickness defined between the inner surface and outer surface. The stent may comprise a plurality of surface textures extending from the stent surfaces, wherein the textures are arranged in a macropattern.
Owner:BVW HLDG
Nanogold film memory alloy esophageal stent and preparing method thereof
ActiveCN105030393ASimplified coating processNo radioactive contaminationStentsTherapeutic coolingNitinol stentTitanium alloy
The invention belongs to the technical field of medical instruments and relates to a nanogold film memory alloy esophageal stent with a photo-thermal therapy function. A nanogold film is prepared by conducting in-situ surface chloroauric acid reduction on a high polymer material with a reduction function used for modifying the surface of a memory alloy wire under the alkaline condition. According to the preparing method, a unique nanogold surface coating method is adopted, the nanogold material is safe and free of toxicity, and the coating process is simple and quick; photo-thermal efficiency is high, and repeated use is realized. The method can be applied to nickel-titanium alloy stents in various shapes and types such as a biliary stent, an intestinal tract stent, a urinary tract stent and a tracheal stent.
Owner:SHANGHAI THERANOSTICS BIOTECH CO LTD
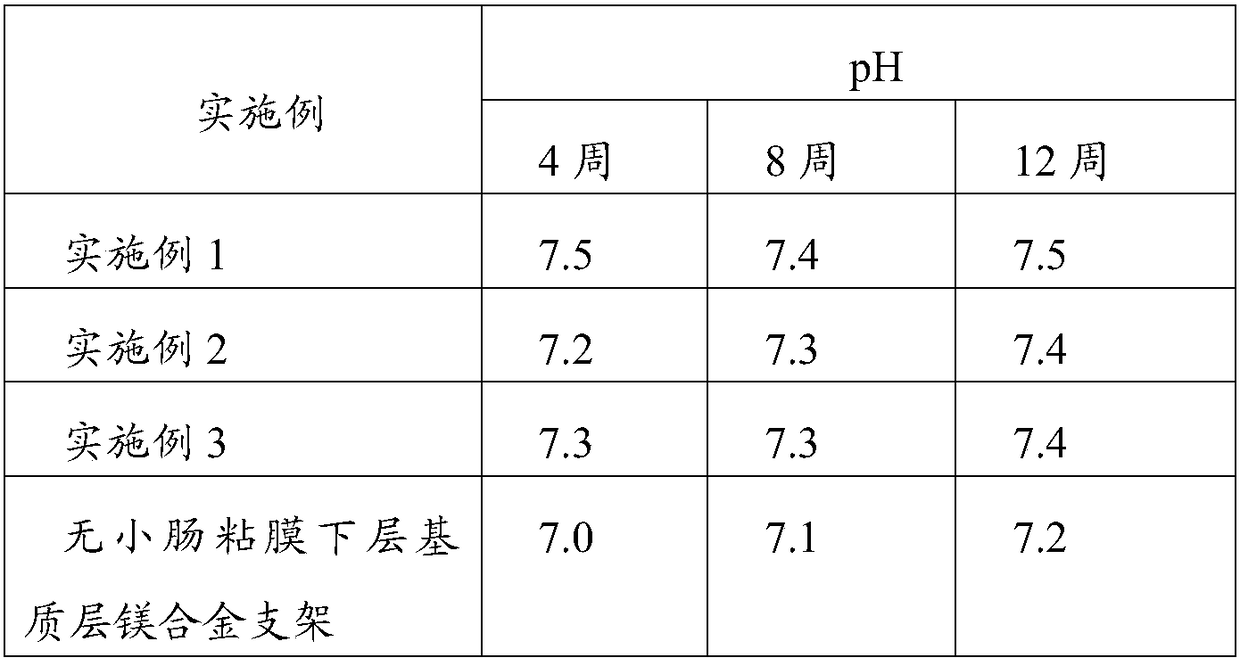
Application of magnesium alloy in preparation of degradable biliary stent
InactiveCN108785755APrevent negative refactoringAvoid damageStentsSurgeryBiliary sludgeStent implantation
The invention relates to the technical field of medical materials and provides a degradable biliary stent. The degradable biliary stent is a magnesium alloy degradable biliary stent. The magnesium alloy degradable biliary stent comprises a plurality of ring-shaped supporting parts distributed in the radial direction and used for radial supporting during ball expansion and a plurality of connectingparts distributed in the axial direction and used for axial connection with the adjacent ring-shaped supporting parts to form a net-like structure. The magnesium alloy degradable biliary stent replaces a non-degradable biliary stent, serves as a ball expansion stent to be implanted at the narrow part of biliary lesion and plays a drainage effect in a support, and the phenomenon can be avoided that the limitations of a stent tube cause complications or the non-degradable biliary stent is difficult to take out. Different from a covered stent, the stent is difficult to displace and has a wider lesion position application range. It is proved through animal experiments that the biliary tracts at the suture parts of experimented animals in an experimental group are smooth and free of biliary sludge or lithogenesis by applying the magnesium alloy degradable biliary stent, and the stent is completely degraded in the eighth week.
Owner:王缨 +1
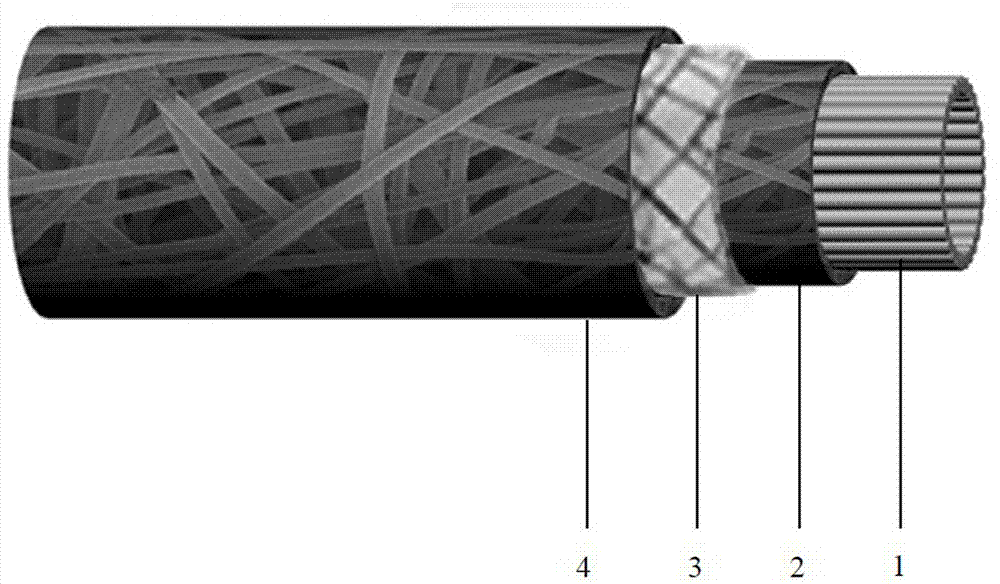
Silver-loaded biliary tract stent with multiple layers of film coatings and preparation method of silver-loaded biliary tract stent
InactiveCN102151338APrevent retrograde infectionImprove patencySurgeryCoatingsStent patencyBiliary stents
The invention relates to the field of medical materials, in particular to a silver-loaded biliary tract stent with multiple layers of film coatings, which comprises a biliary tract stent body, wherein a multiple layers of film coatings which are alternately arranged according to chitosan nano silver layers and heparin layers are coated on the surface of the biliary tract stent body, the total layer quantity of the multiple layers of film coatings is not less than 4, and the total thickness of the multiple layers of film coatings is between 100 and 2000nm. The invention also provides a preparation method of the silver-loaded biliary tract stent with multiple layers of film coatings, which comprises the steps of: dip-coating the biliary tract stent body in a heparin solution and then dip-coating in a chitosan-loaded nano silver solution, and circularly repeating the steps. The silver-loaded biliary tract stent with multiple layers of film coatings, prepared by utilizing the preparation method disclosed by the invention, has the functions of resisting bacteria, virus and mycete; and due to the adoption of the silver-loaded biliary tract stent, the unobstructed period of the biliary tract stent can be prolonged and the inverse infection of the biliary tract can be prevented.
Owner:ZHEJIANG UNIV
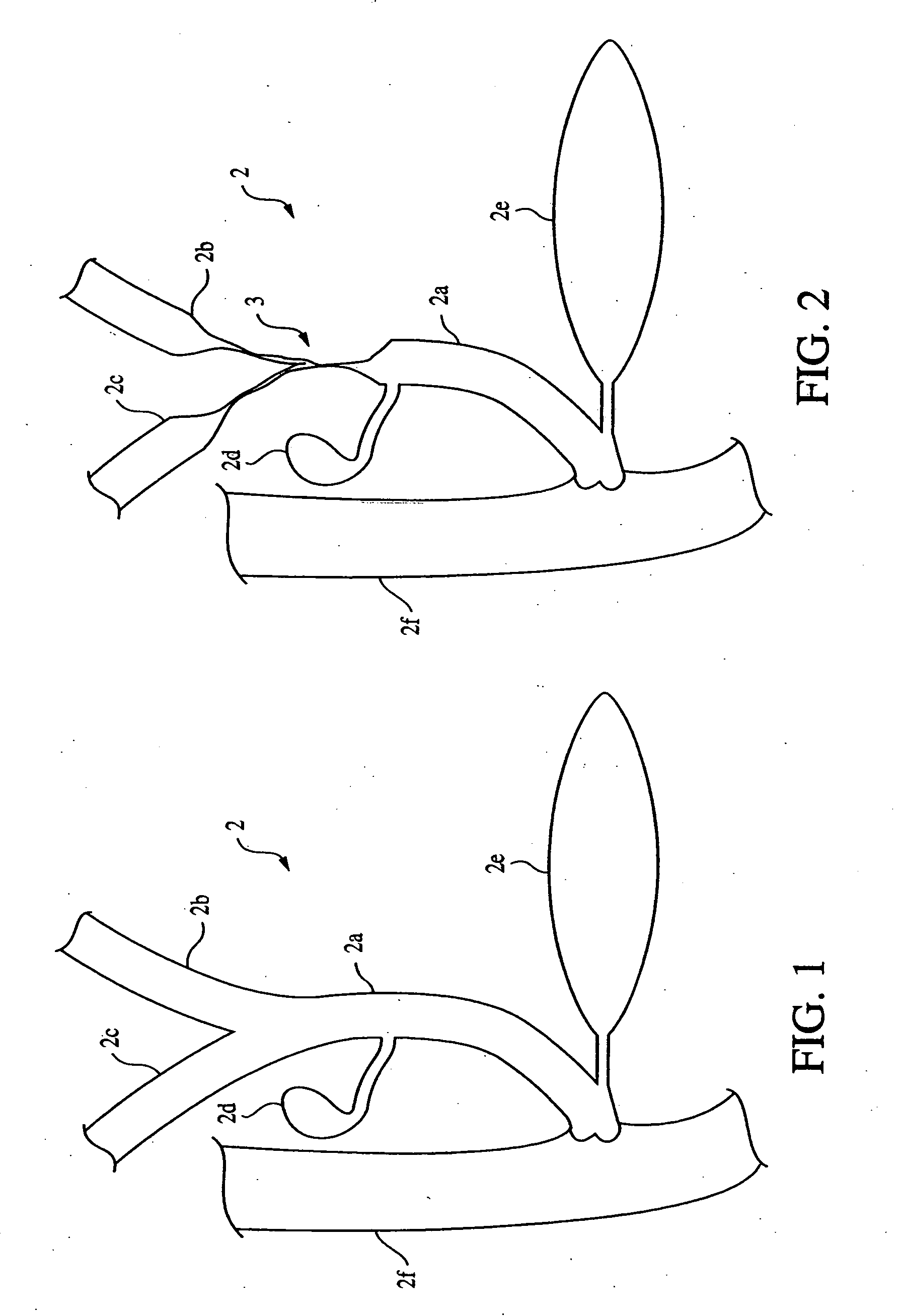
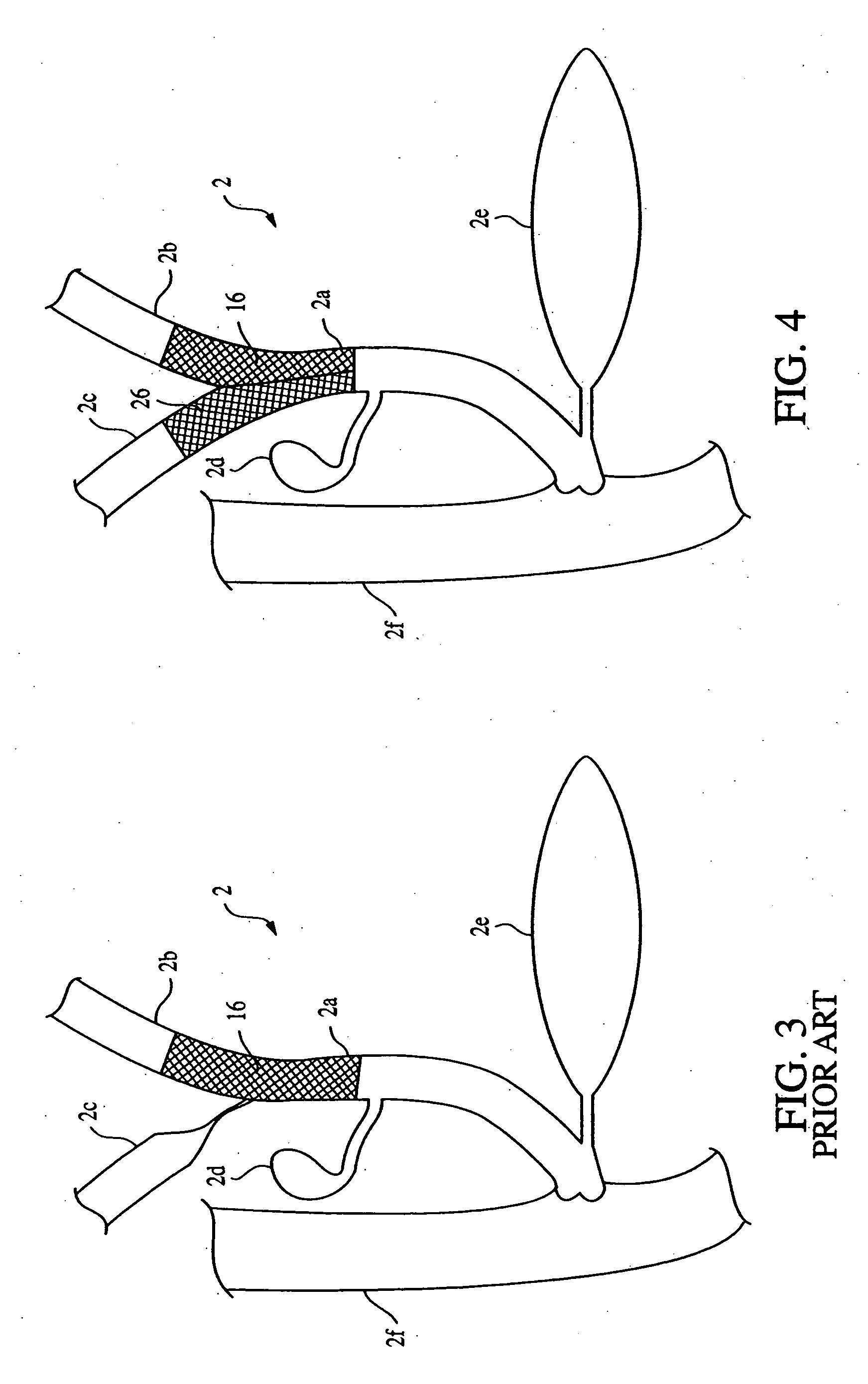
Anti-regurgitation biliary stent
The invention relates to an anti-regurgitation biliary stent which is provided with a stent body and side wings. The biliary stent is further provided with an anti-regurgitation valve which is in a nipple shape and provided with a fixing ring, a cannula and a capsule bag with a releasing crack. The fixing ring coordinates with an annular groove in the tail end of the stent body to enable the anti-regurgitation valve to be fixed to the stent body. The cross section of the groove is in an arc shape, and the central angle of the groove is 180-240 degrees. The releasing crack is in a Y shape or a cross shape. Due to the fact that the anti-regurgitation valve is arranged on the biliary stent, and the spherical capsule bag with the releasing crack is arranged on the anti-regurgitation valve, the phenomena of biliary intestinal regurgitation and biliary tract infection can be reduced, the unobstructed period of the plastic biliary stent can be prolonged possibly, and the survival quality of patients is improved greatly; the junction between the anti-regurgitation valve and the stent body is firm, and the anti-regurgitation valve is not prone to loosening and disengaging; the whole biliary stent is simple in structure, convenient to use and suitable for being popularized in clinic.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
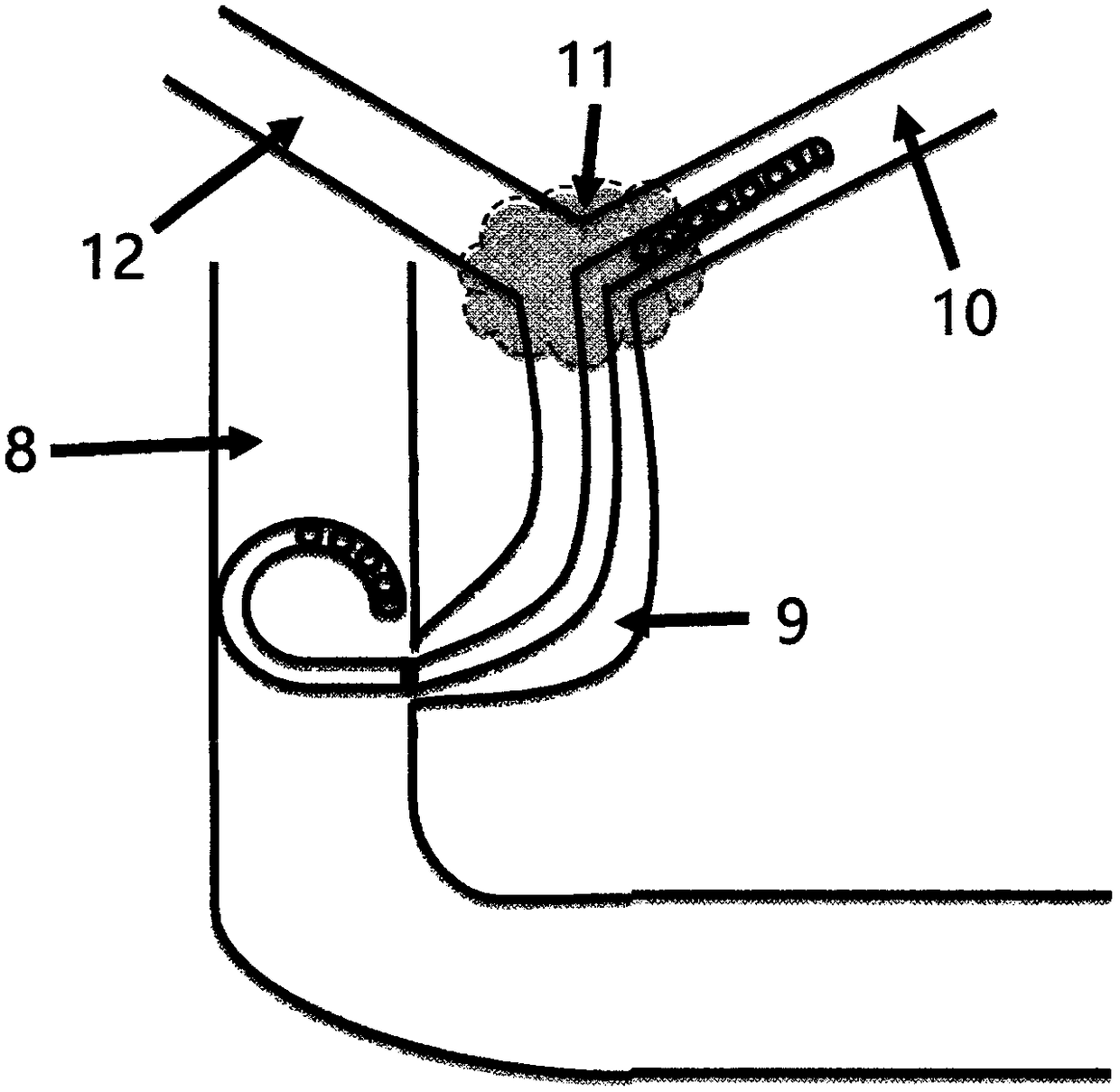
Anti-regurgitation gallbladder-intestine stent
The invention provides an anti-regurgitation gallbladder-intestine stent.The anti-regurgitation gallbladder-intestine stent comprises a tubular intestine stent body and a tubular biliary stent body.An inlet of the intestine stent body is detachably connected with an outlet of the biliary stent body.An outlet of the intestine stent body is provided with an anti-regurgitation petal.The petal is in a to-be-bloomed flower bud shape and comprises multiple petal pieces stacked in the circumferential direction of the intestine stent body.The fixed ends of the petal pieces are fixed to the end face of the outlet of the intestine stent body.The free ends of the petal pieces obliquely draw together towards the center of the intestine stent body.The adjacent petal pieces are in press fit, and an outwards-protruding airtight petal chamber is defined at the outlet of the intestine stent body.The anti-regurgitation gallbladder-intestine stent is easy to operate, high in success rate and small in trauma, the petal can only bloom forwards to form an outlet, gallbladder-intestine regurgitation can be effectively prevented, and regurgitation cholangitis is effectively reduced.
Owner:ZHEJIANG UNIV
Drug-loaded stent with ultrasonic intelligent controlled release
InactiveCN102319453AWith ultrasonic intelligent controlled releaseConfirm new roleSurgeryCoatingsHuman bodyAnticarcinogenic Effect
The invention relates to a stent coating with ultrasonic intelligent controlled release and a preparation method and an application thereof. The invention also provides a drug-loaded stent with ultrasonic intelligent controlled release and a preparation method and an application thereof. The advantages of the invention are that: a new stent coating with the effect of ultrasonic intelligent controlled release is successfully constructed and synthesized; ultrasound is first found and confirmed to have control effect on the temperature-sensitive release of loaded drugs; and a new method is provided for future drug intelligent controlled release; a drug-loaded biliary tract stent with ultrasonic intelligent drug controlled release is synthesized, and a possible and feasible treatment means is provided for the treatment of malignant tumors of hollow organs; ultrasound is first found and confirmed to have significant effect on promoting the release of drugs coated by a temperature-sensitive material by changing the spatial structure of the temperature-sensitive material. By using ultrasound, the effect of drug intelligent controlled release at a designated site is achieved; local anticancer effect is brought into play; the toxic and side effect of anticancer drugs on important human tissue is reduced; and high clinical application value is provided.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
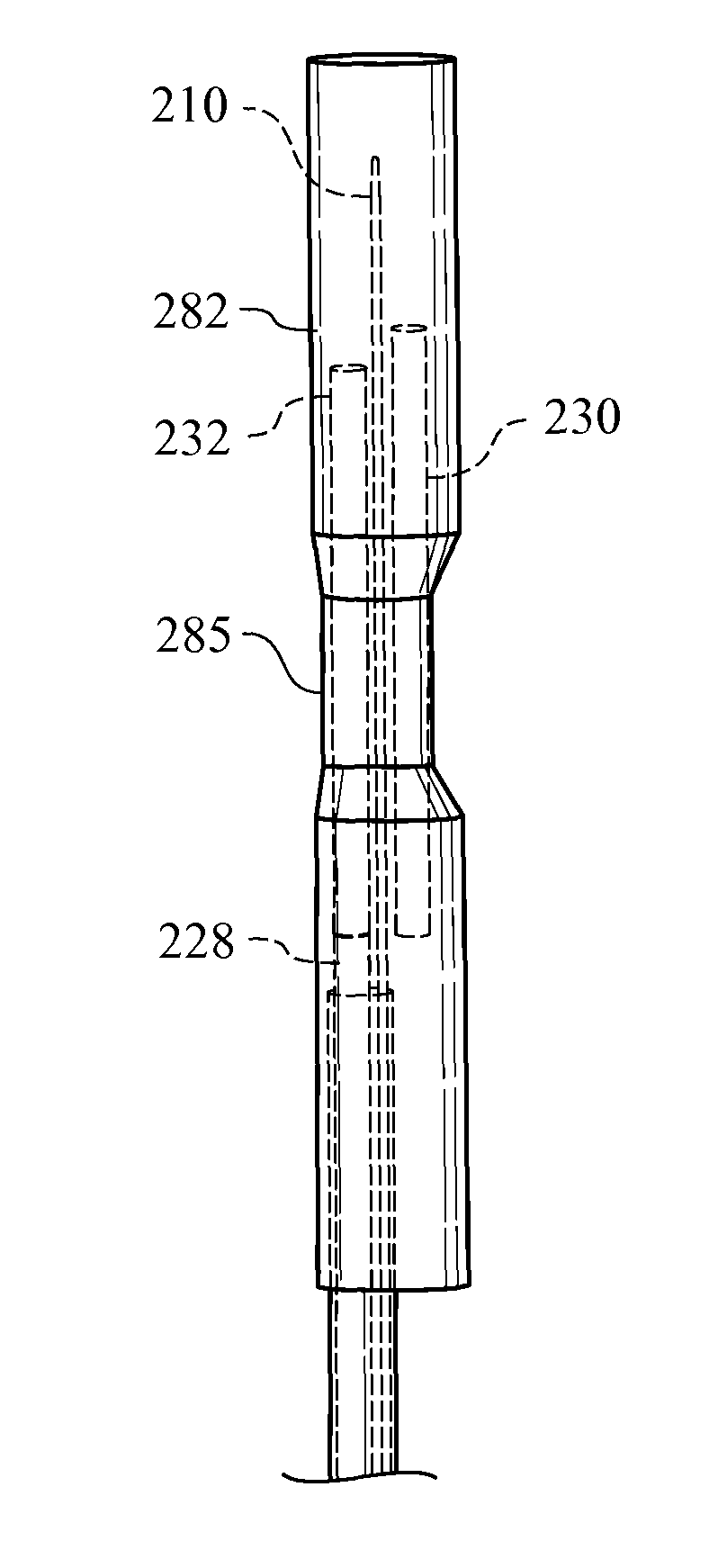
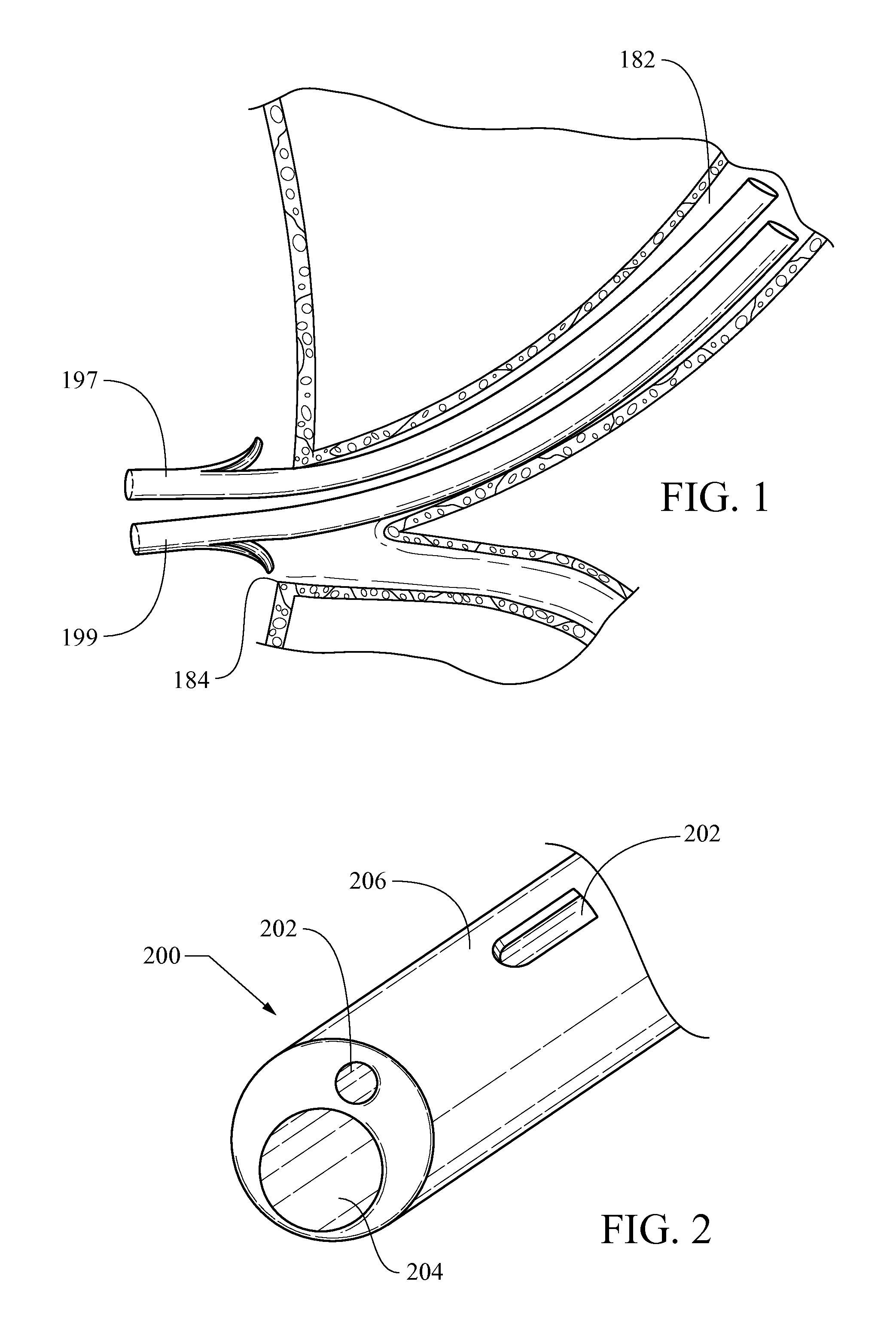
Method of placing multiple biliary stents without re-intervention, and device for same
A dual lumen catheter may be provided with one or more stents in a stent-deployment lumen and a wire guide disposed through a wire guide lumen. The wire guide may be directed to a target site in a patient body such as a biliary stricture. The catheter may be directed along the wire guide until it is in or adjacent the target site. A distalmost stent may be advanced out a distal side-facing aperture of the stent-deployment lumen into the target site by a pusher member that advances the stent or that holds the stent in place while the catheter is proximally withdrawn from around the stent. With the wire guide remaining substantially in place, the stent-deployment lumen can be reoriented and the steps repeated to place a second (and, if desired, subsequent) stent(s) next to—and generally parallel with—the first stent.
Owner:COOK MEDICAL TECH LLC
Magnesium alloy biliary stent and preparation method thereof
InactiveCN108354698AGood biocompatibilityNon-immunogenicStentsAnodisationTest sampleSmall intestine mucous membrane
The invention discloses a magnesium alloy biliary stent and a preparation method thereof. The magnesium alloy biliary stent comprises an extensible magnesium alloy stent body, wherein small intestinemucous membrane lower-layer matrix layers are arranged on the inner wall and the outer wall of the magnesium alloy stent body. The preparation method comprises the following steps that the small intestine mucous membrane lower-layer matrix layers are prepared; the magnesium alloy stent body is pretreated to remove oxide on the surface of the stent body; electrolyte is prepared, wherein the magnesium alloy stent body is put in micro-arc oxidized electrolyte, the magnesium alloy stent body serves as an anode, and a stainless-steel electrolytic tank serves as a cathode to perform micro-arc oxidization treatment; a test sample is post-treated; the prepared small intestine mucous membrane lower-layer matrix layers are suture or bonded on the inner layer and the outer layer of the magnesium alloy stent body respectively to obtain the magnesium alloy biliary stent. The magnesium alloy biliary stent has good biocompatibility, can be biodegraded into a nontoxic product and has no immunogenicity.
Owner:秦高平
Degradable radioactive biliary stent and method for producing the same
The invention relates to a radioactive bile duct support which can be degraded, used to treat cancer, wherein it is formed by the common bile duct support woven from NiTi alloy lines and the degradable belly film made from radioactive material covering the support. And its production comprises (1), using NiTi alloy lines to weave the bile duct support; (2), plating belly layer on the support, emerging it in crosslinker; (3), freezing and drying; (4), before using, using epoxyethane gas to disinfect it. The invention can treat and restrain the membrane increment and cancer growth, without hurting normal organism. And the epoxyethane, dope and chitose of carrier can be degraded.
Owner:六安市济民医药科技有限公司
Method of placing multiple biliary stents without re-intervention, and device for same
A dual lumen catheter may be provided with one or more stents in a stent-deployment lumen and a wire guide disposed through a wire guide lumen. The wire guide may be directed to a target site in a patient body such as a biliary stricture. The catheter may be directed along the wire guide until it is in or adjacent the target site. A distalmost stent may be advanced out a distal side-facing aperture of the stent-deployment lumen into the target site by a pusher member that advances the stent or that holds the stent in place while the catheter is proximally withdrawn from around the stent. With the wire guide remaining substantially in place, the stent-deployment lumen can be reoriented and the steps repeated to place a second (and, if desired, subsequent) stent(s) next to—and generally parallel with—the first stent.
Owner:COOK MEDICAL TECH LLC
'J' type biliary stent for drainage of high biliary obstruction
PendingCN108451677AImprove practicalityAvoid displacementStentsMedical devicesInsertion stentBiliary obstructions
The invention provides a 'J' type biliary stent for drainage of high biliary obstruction, which comprises: a bile duct part arranged in a common bile duct and an intestinal part arranged in a duodenum. The intestinal part is integrally connected with the bile duct part. A large 'C' type structure is formed in the intestinal part. By arranging the bile duct part and the intestinal part and settingthe intestinal part as a large 'C' type structure, the intestinal part can form a 'C' type ring at the descending part of duodenum, which effectively avoids the situations of movement of the long frame for drainage of high-level biliary obstruction to the bile duct and slip of the duodenum. As a result, the drainage effect is more stable and reliable, and the practicability of the 'J' type biliary stent for drainage of high biliary obstruction is improved. The manufacture is simple, which facilitates the popularization and application in the market.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
Claw-shaped bile duct and pancreatic stent taking-out instrument
The invention relates to the field of medical instruments, and particularly relates to a claw-shaped bile duct and pancreatic stent taking-out instrument, which comprises an outer sheath, a piston cavity is formed in the left end part of the outer sheath tube; the lower end of the piston cavity communicates with a conduit; the other end of the catheter penetrates out of the right end of the outersheath tube and is provided with an air bag assembly, a cavity ejecting piece is arranged at the upper end of the piston cavity; the lower side of the cavity ejecting piece is connected with a pistonpiece matched with the piston cavity through a spring; a connecting rod is arranged on the upper side of the piston part, the upper end of the connecting rod penetrates through and penetrates out of the cavity top part, a moving disc is integrally arranged at the upper end of the connecting rod, multiple sets of taking-out parts are evenly, obliquely and rotationally connected to the upper side ofthe moving disc, claw hooks are obliquely arranged at the top ends of the taking-out parts, and notches of the claw hooks face outwards; according to the invention, a biliary stent is hooked and taken out from the inner side of the biliary stent through the claw hooks, so that the duct at the imbedding part is prevented from being expanded, the difficulty of hooking the biliary stent by the clawhooks is reduced, and the lesion of the pipeline is prevented from being damaged.
Owner:CHANGZHOU NO 2 PEOPLES HOSPITAL
Memory alloy esophageal stent modified by nano copper sulfide coating and preparation method of memory alloy esophageal stent
ActiveCN113499483AStable in natureNot easy to decomposeStentsPharmaceutical delivery mechanismUrethral stentsNitinol stent
The invention belongs to the technical field of medical instruments, and relates to a memory alloy esophageal stent with a uniform nano copper sulfide film and an efficient photo-thermal physiotherapy function and a preparation method of the memory alloy esophageal stent. The stent is prepared by reducing dopamine under an alkaline condition to obtain a polydopamine-coated memory alloy stent, and then efficiently adsorbing copper ions through polydopamine under a heating condition to grow copper sulfide in situ. According to the method, a unique method for in-situ growth of nano CuS particles on the surface of the stent is adopted, the nano CuS material is safe and non-toxic, and the growth process is simple and rapid; photo-thermal conversion is efficient, and repeated use can be achieved; the method can be applied to nickel-titanium alloy stents of various shapes and other types, such as biliary tract stents, intestinal tract stents, urinary tract stents and tracheal stents.
Owner:FUZHOU UNIV
Suspension-type regurgitation-resistant biliary stent
The invention provides a suspension-type regurgitation-resistant biliary stent. The stent comprises a biliary duct part for being arranged in the common bile duct and an intestinal part for being arranged in the duodenum, the total length of the biliary stent is smaller than or equal to 25 cm, the length of the biliary duct part is larger than or equal to 10 cm, and the length of the intestinal part is larger than or equal to 13 cm. The biliary stent is placed in the human body and more easily accepted by a patient, complications such as perforation are not easily caused, and when the intestinal part is placed in the duodenum and can reach the horizontal part in the duodenum, the countercurrent condition of content in the duodenum is effectively reduced; in addition, a bending part is located between a first connection segment and a second connection segment, so that a preset angle is formed between the first connection segment and the second connection segment, in this way, the intestinal part can better adapt to deformation of the enteric cavity, the biliary stent is conveniently placed and prevented from being in contact with the intestinal mucosa, and the discomfort of the human body after the stent is placed in the human body is relieved.
Owner:PEKING UNIV THIRD HOSPITAL
Development type degradable repair biliary tract stent
PendingCN105944153AGood biocompatibilityDegradation time controllableSurgeryBiliary tract reconstructionPostoperative cholangiography
The invention relates to a development type degradable repair biliary tract stent. The development type degradable repair biliary tract stent comprises, by weight, 50-100 parts of degradable medical materials A, 0.1-50 parts of healing repair materials B, 0.001-0.05 part of developers C, 0.001-0.05 part of anti-biliary tract-tumor medicines and / or anti-gallstone medicines D and 0.001-0.05 part of vitamins. The development type degradable repair biliary tract stent has the advantages that the development type degradable repair biliary tract stent is excellent in biocompatibility and stable and controllable in degradation time, and the morphology and the locations of implant materials can be quickly judged; decompression support and drainage effects can be realized in complicated biliary tract reconstruction procedures, and the development type degradable repair biliary tract stent is favorable for biliary tract healing repair, and can bring convenience for observing bile conditions, postoperative cholangiography effects and the like.
Owner:青岛蓝博云健康产业发展有限公司
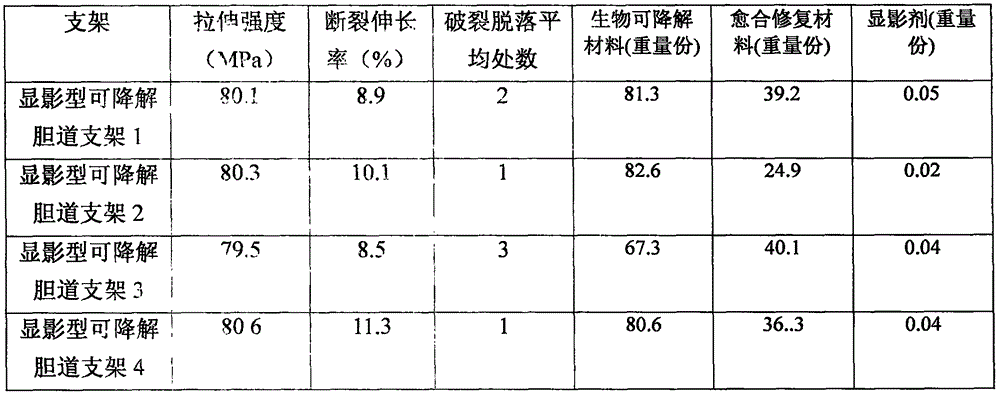
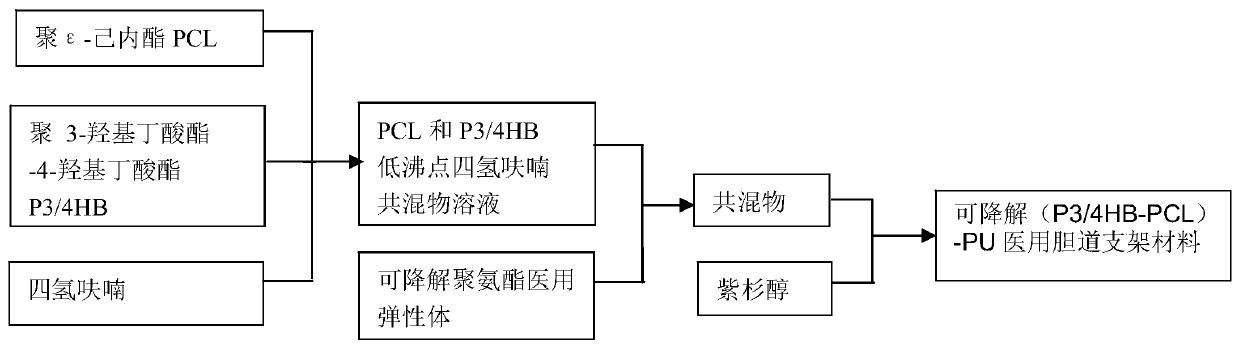
Degradable (P3/4HB-PCL)-PU medical biliary stent material and preparation method thereof
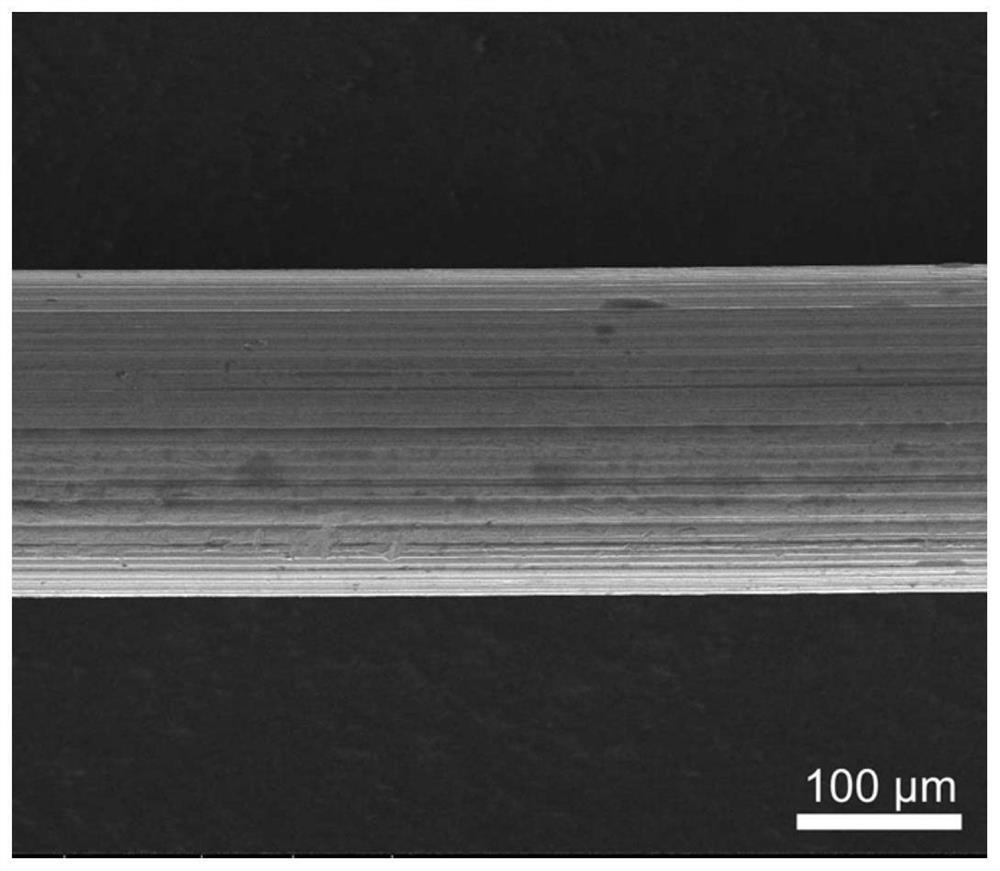
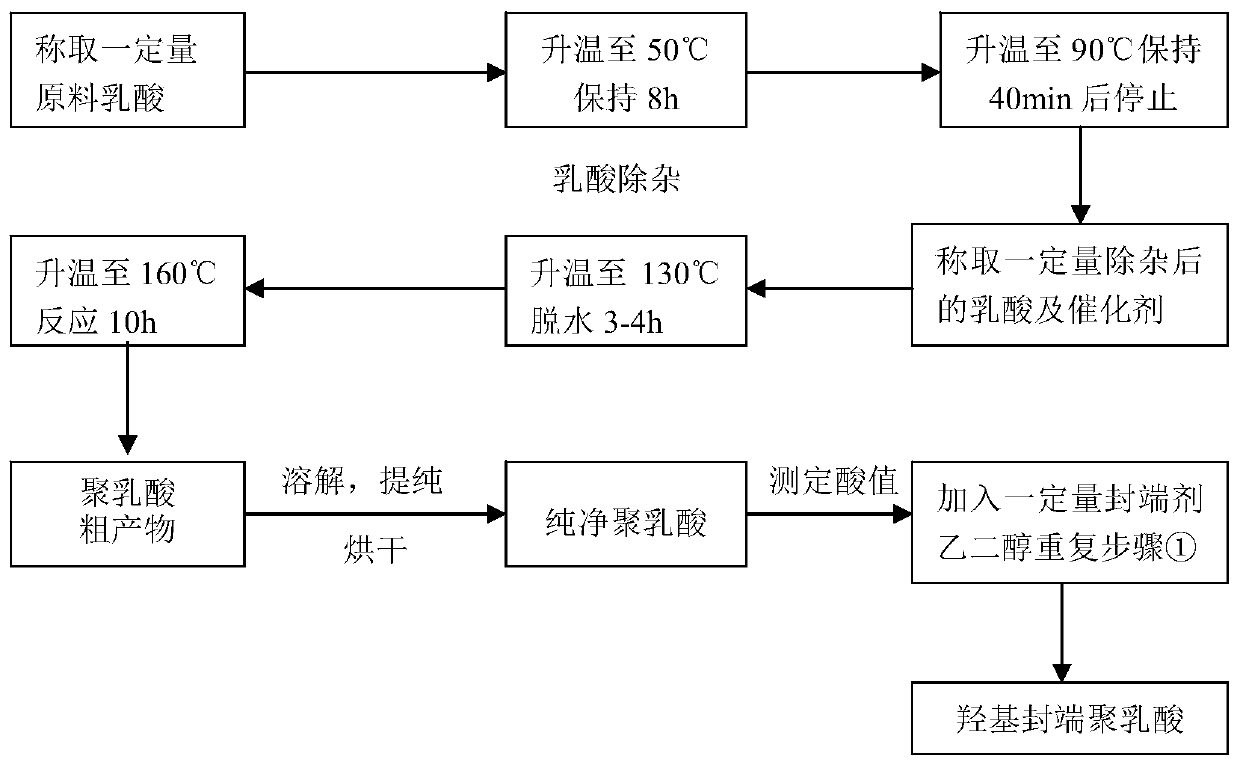
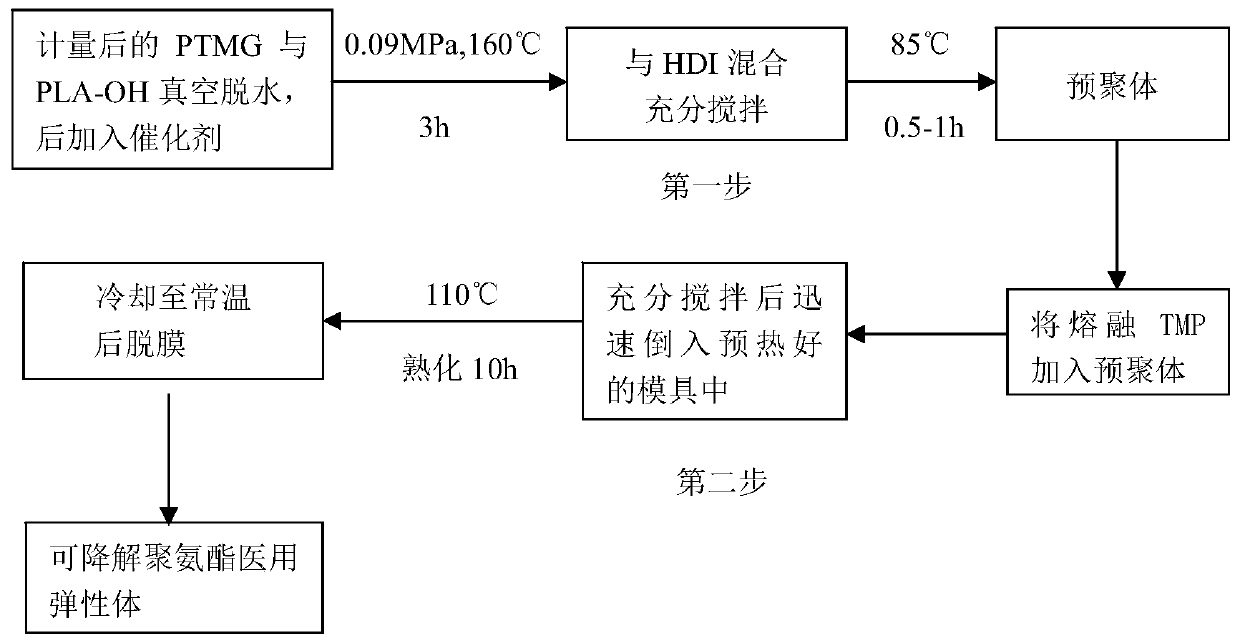
The present invention discloses a degradable (P3 / 4HB-PCL)-PU medical biliary stent material and a preparation method thereof. The material comprises polytetrahydrofuran ether diol (PTMG), hydroxyl terminated polylactic acid (PLA-OH), hexamethylene diisocyanate (HDI), poly-epsilon-caprolactone (PCL), poly 3-hydroxybutyrate-4-hydroxybutyrate (P3 / 4HB), paclitaxel, lactic acid, non-toxic catalysts, dichloromethane, catalysts and a chain extender. The method comprises the following steps: (1) lactic acid impurity removal; (2) hydroxy-terminated polylactic acid synthesis by direct condensation polymerization; (3) degradable polyurethane medical elastomer synthesis; and (4) degradable (P3 / 4HB-PCL)-PU medical biliary stent material preparation. The degradable TAXOL-(P3 / 4HB-PCL)-PU nano-biliary stent is formed by a special soluble core-shaft-assisted coaxial electrospinning technology. The stent has biocompatibility, safety, drug controlled release and benign biliary stricture prevention effect.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
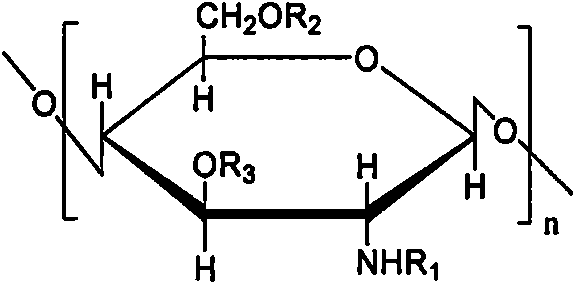
Absorbable tubular stent and preparation method and application thereof
InactiveCN105214144AHigh mechanical strengthGood grip rebound performanceSurgeryUrethral stentsEmbolization Therapy
The invention discloses an absorbable tubular stent. The absorbable tubular stent is characterized in that the tubular stent is made by blending of polylactic acid and acylated chitosan, and the wall of the tubular stent is provided with or without a porous structure or pattern structure. Due to addition of the acylated chitosan, local acidity generated by the polylactic acid in degradation can be regulated, and oligosaccharides and monosaccharide obtained by degradation of the acylated chitosan can be absorbed and used by organisms, so that high biocompatibility, degradability and absorbability are achieved; the tubular stent has excellent mechanical strength and press resilience. The absorbable tubular stent can be planted in vivo by surgeries to serve as an intravascular stent and is applicable to treatment of embolism or blood vessel lumina narrowing caused by vascular diseases; the absorbable tubular stent can also serve as a biliary stent to be applied to treatment of cholestasis and pancreaticobiliary obstruction caused by pancreatobiliary neoplasms; the absorbable tubular stent can further serve as a urethral stent to be applied to treatment of dysuria resulted from urethral compression caused by prostatic hyperplasia. The absorbable tubular stent can be degraded and absorbed in vivo finally along with lesion repair, in-vivo long-term retention is avoided, and the absorbable tubular stent has a promising market prospect.
Owner:OCEAN UNIV OF CHINA
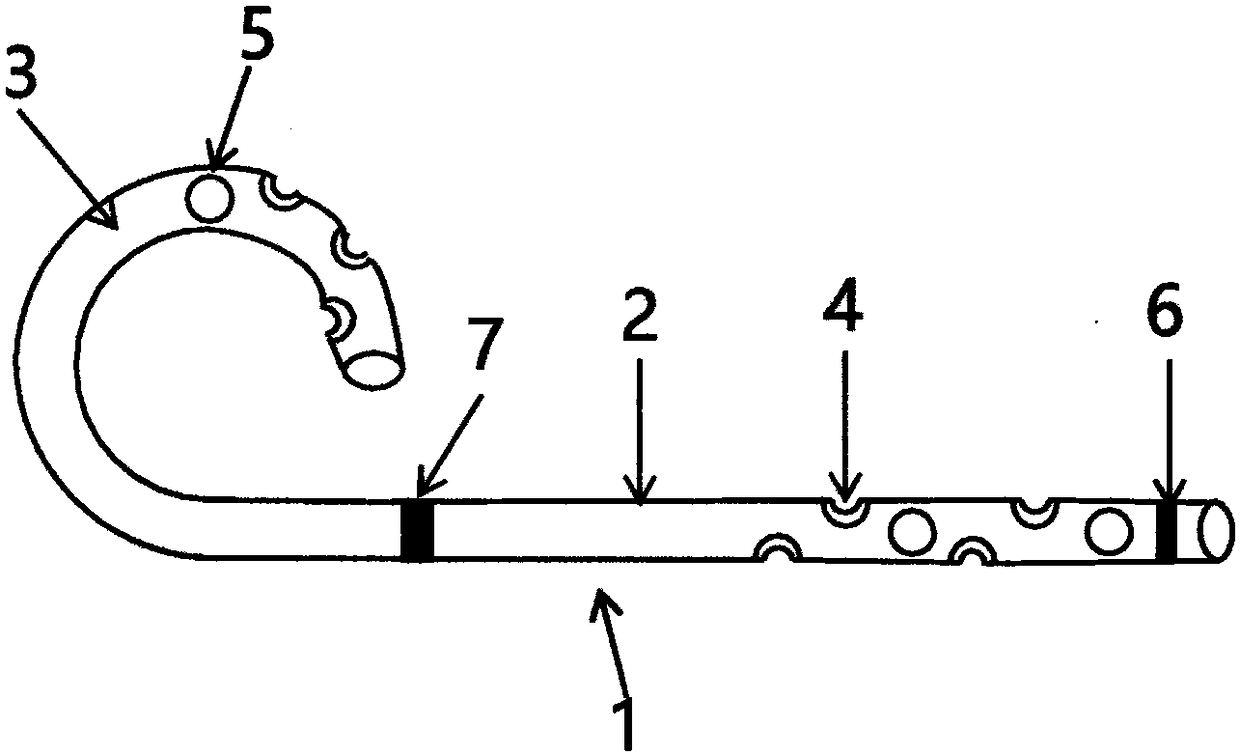
Recyclable biliary stent
The invention discloses a recyclable biliary stent. The biliary stent comprises a stent main body, and a lead wire connected with the stent main body, wherein the stent main body is of a spiral shape; an external thread is arranged at the near end of the stent main body, and an internal thread is arranged at the far end of the lead wire; and the lead wire and the stent main body are in threaded connection. The stent main body is of a spiral shape, and is connected with the lead wire, so that the stent main body can be taken out conveniently through the lead wire extending outside a human body.
Owner:姜在波
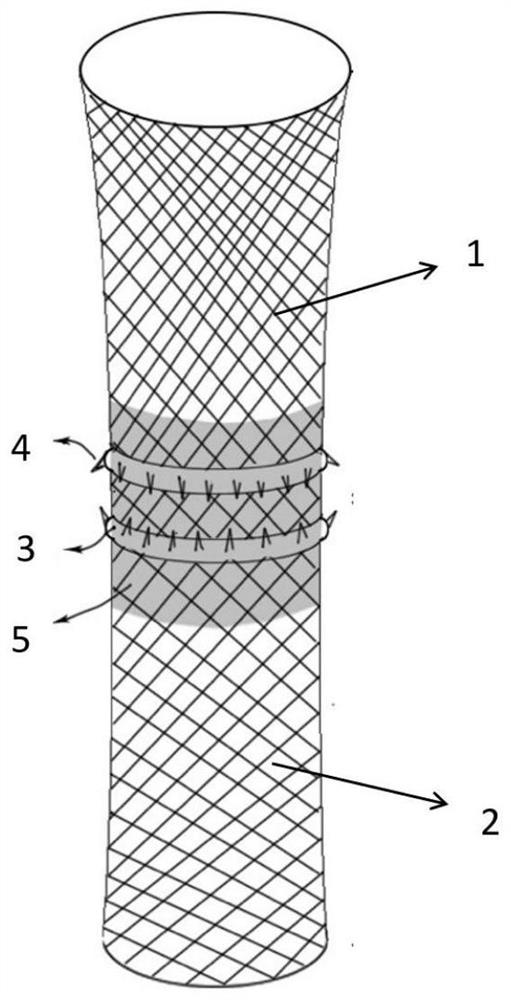
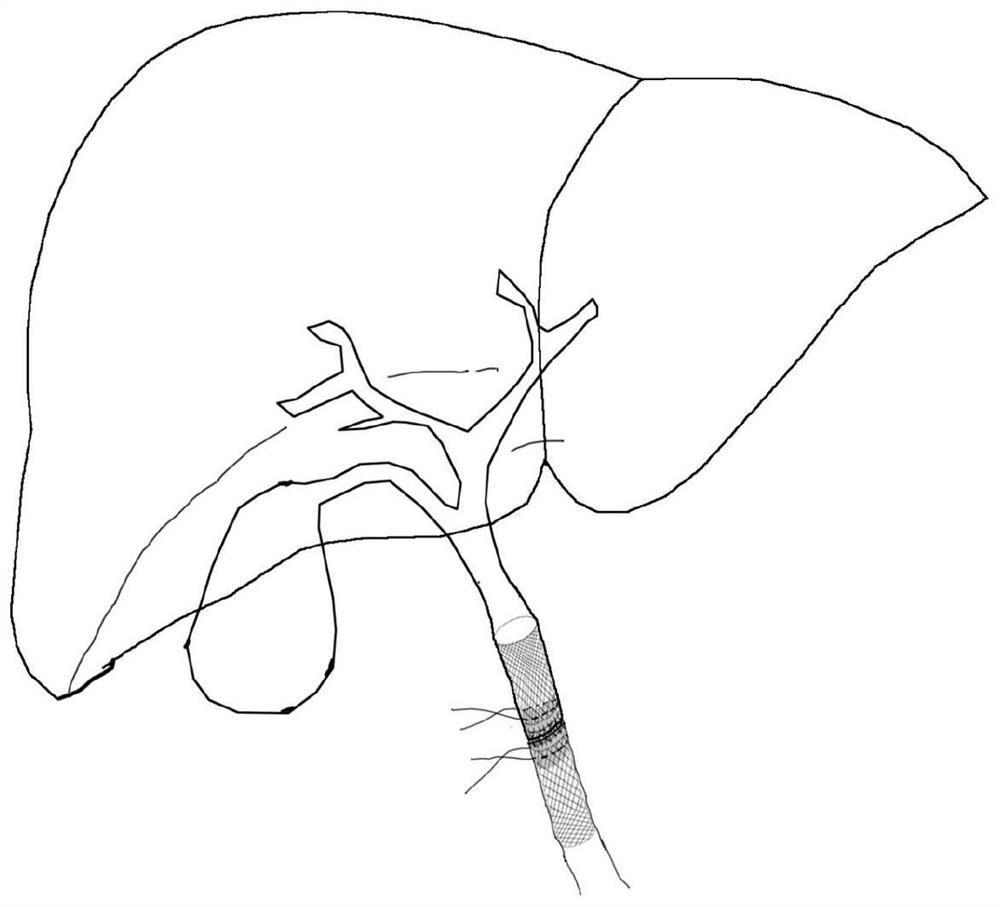
Biliary stent
PendingCN113397775AEnable Personalized TreatmentEasy to fixStentsAdditive manufacturing apparatusBiliary stentBiocompatibility
The invention aims to provide a biliary stent and solves the problems that a biliary stent is non-degradable, poor in matching, prone to moving and poor in biocompatibility in the liver transplantation process. The biliary stent is used for biliary support of an anastomosis part after liver transplantation and comprises a first stent body, a second stent body, a fixing part and a covering film; the first stent body and the second stent body are of a net-shaped hollow structure integrally formed through 3D printing; the outer diameter of the first stent body and the outer diameter of the second stent body are the same as the inner diameter of a donor biliary tract and the inner diameter of a receptor biliary tract respectively; the fixing part is arranged on the outer surface of the joint of the first stent body and the second stent body; and the covering film is arranged on the inner surface of the joint of the first stent body and the second stent body.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com