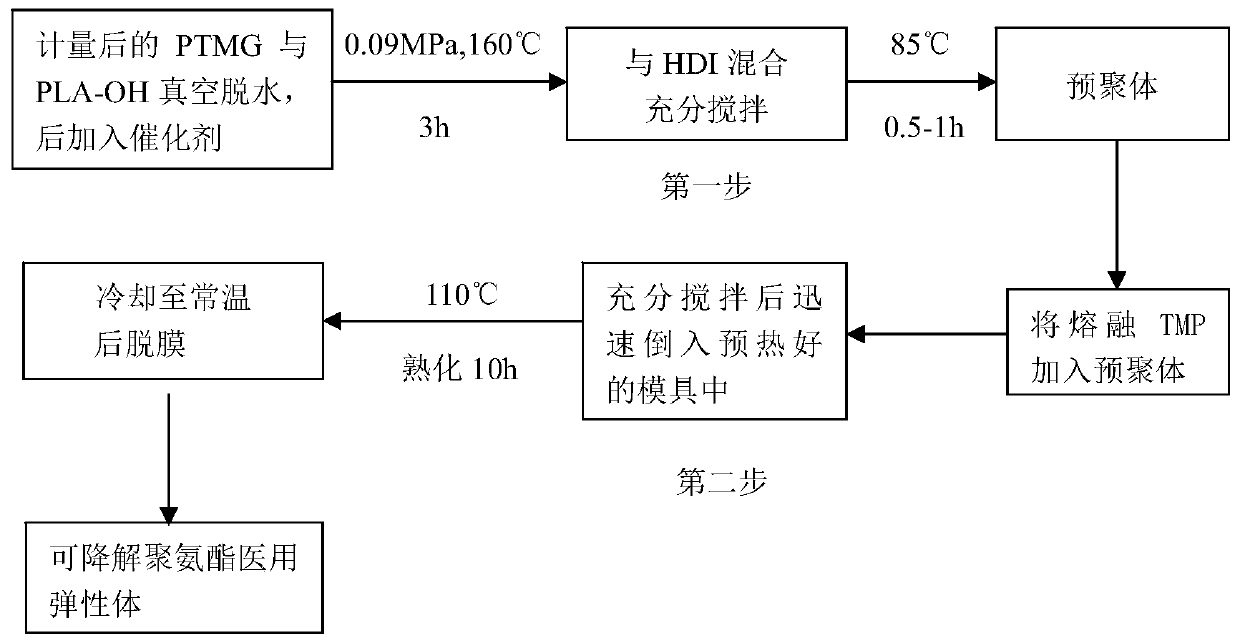
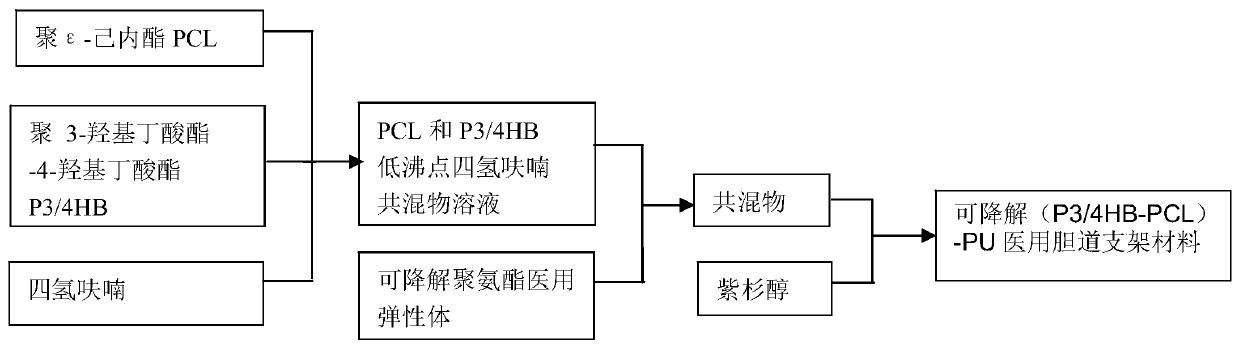
Degradable (P3/4HB-PCL)-PU medical biliary stent material and preparation method thereof
A stent material, -PU technology, used in medical science, surgery and other directions, can solve the problems of high cost of metal stents, inability to provide radial support for the bile duct, no degradability, etc., and achieve good biocompatibility and degradability. The effect of preventing benign biliary stricture and biliary sludge deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
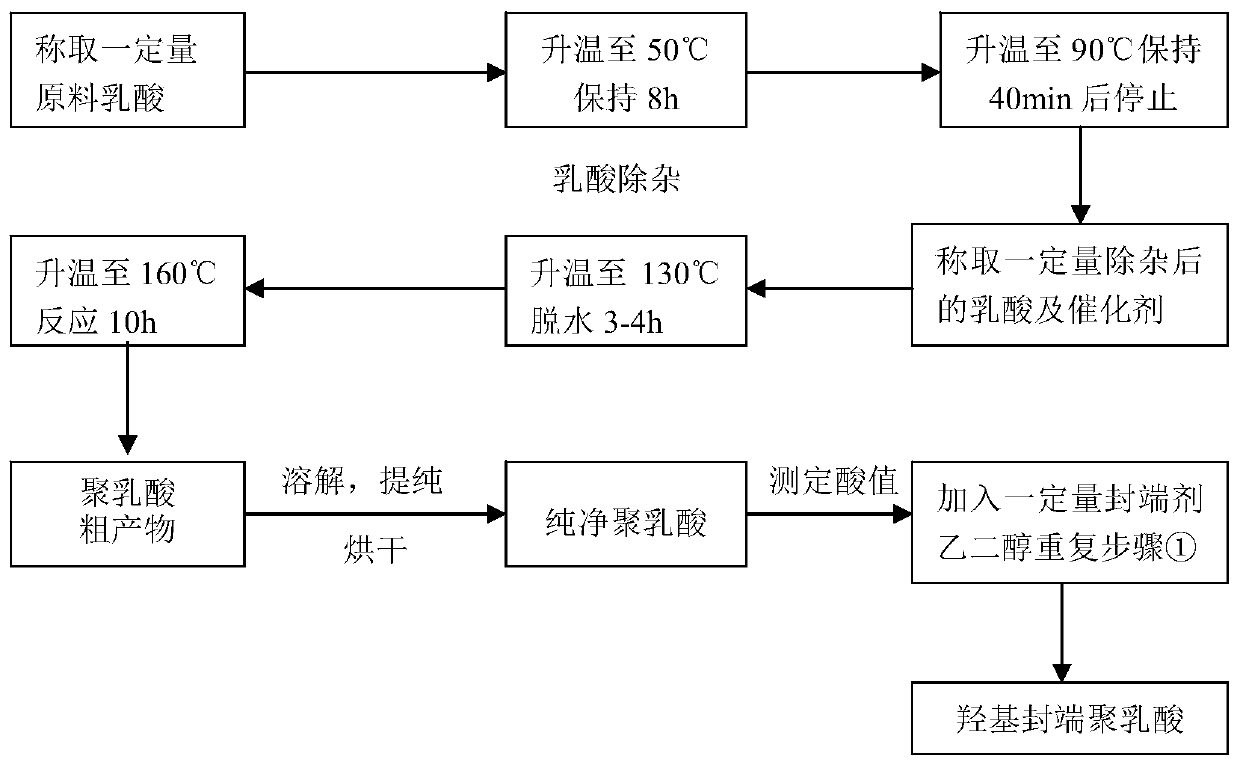
[0039]The preparation method step of degradable (P3 / 4HB-PCL)-PU medical biliary tract stent material of the present invention is as follows:
[0040] 1) Lactic acid removal
[0041] Put the lactic acid into the container, under the protection of nitrogen, raise the temperature to 30-60°C under the vacuum degree of 0.01-0.1MPa and react for 7-9 hours, then raise the temperature to 70-100°C at a heating rate of 2-10°C / 5-15min , kept for 35 to 50 minutes to obtain a colorless or light yellow transparent liquid;
[0042] 2) Synthesis of hydroxyl-terminated polylactic acid by direct polycondensation method
[0043] a. Mix and stir the lactic acid dehydrated under reduced pressure with a mass ratio of 90 to 120 parts and 0.3 to 0.9 parts of non-toxic catalyst (zinc lactate, zinc acetate, zinc sulfate or taurine). Under 0.1MPa vacuum degree, raise the temperature to 120-150°C in stages, stir and dehydrate for 3-4 hours, then raise the temperature to 140-170°C at a rate of 2-10°C / 25...
Embodiment 1
[0054] 1) Under the protection of nitrogen, under the vacuum degree of 0.05MPa, heat the lactic acid in the container to 40°C for 8 hours, then raise the temperature to 80°C at a heating rate of 2°C / 5min, and keep it for 40min to obtain a colorless or light yellow transparent liquid;
[0055] 2) Synthesis of PLA-OH by direct polycondensation method
[0056] a. Mix and stir 100 parts of lactic acid dehydrated under reduced pressure and 0.6 parts of zinc lactate, a non-toxic catalyst. Under the protection of nitrogen, the temperature is raised to 140 ° C in stages under a vacuum of 0.6 MPa for 3 hours, and then dehydrated at 8 ° C / 30 min. Raise the temperature to 160°C and continue the reaction for 10 hours. Pour the product into a tray lined with a polytetrafluoroethylene film while it is hot, and pulverize it after cooling to room temperature to obtain a milky white or light yellow-brown crude product of polylactic acid;
[0057] b. Dissolve 750 parts of the crude polylacti...
Embodiment 2
[0064] 1) Under the protection of nitrogen, under the vacuum degree of 0.01MPa, heat the lactic acid in the container to 60°C and react for 7 hours, then raise the temperature to 100°C at a heating rate of 10°C / 10min, and keep it for 35min to obtain a colorless or light yellow transparent liquid;
[0065] 2) Synthesis of hydroxyl-terminated polylactic acid by direct polycondensation method
[0066] a. 120 parts of lactic acid dehydrated under reduced pressure, 0.9 parts of non-toxic catalyst zinc acetate, zinc sulfate or taurine) were mixed and stirred, and under the protection of nitrogen, the temperature was raised to 150 ° C in stages under a vacuum of 0.01 MPa and stirred for 3 hours. , then heated up to 140°C at a rate of 2°C / 40min for continuous reaction for 9 hours, poured the product into a tray lined with a polytetrafluoroethylene film while it was hot, and pulverized it after cooling to room temperature to obtain milky white or light yellow-brown crude polylactic aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com