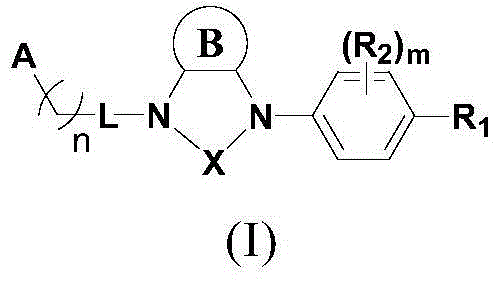
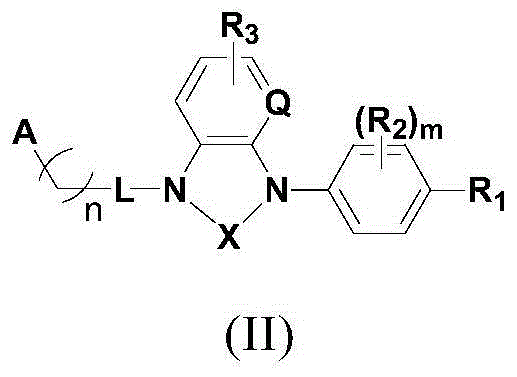
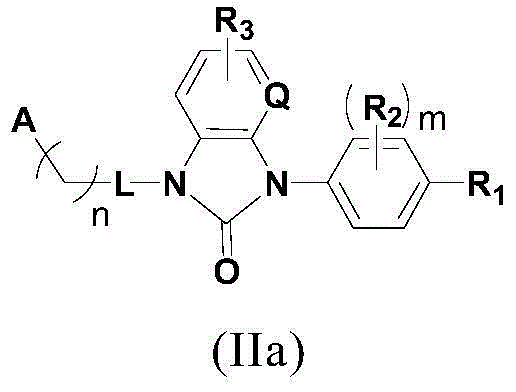
Compound used as RORgamma conditioning agent
A technology of compounds, solvates, applied in the field of compounds as ROR gamma modulators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0201] Preparation of compounds of the present invention
[0202] The following reaction schemes illustrate the preparation of compounds of formula I of the present invention.
[0203] It should be understood by those skilled in the art that in the following description, combinations of substituents are permissible only when such combination can result in a stable compound.
[0204] Those skilled in the art will also understand that in the methods described below, the functional groups of intermediate compounds may need to be protected by appropriate protecting groups. Such functional groups include hydroxyl, amino, amidino, guanidino, mercapto and carboxyl groups. Suitable hydroxy protecting groups include trialkylsilyl or diarylalkylsilyl groups (eg tert-butyldimethylsilyl, tert-butyldiphenylsilyl or trimethylsilyl) , tetrahydropyranyl, benzyl, etc. Suitable protecting groups for amino, amidino and guanidino include tert-butoxycarbonyl, benzyloxycarbonyl and the like. S...
Embodiment 1
[0226] Example 1: 4-(3-(2,6-dichlorobenzoyl)-7-fluoro-2-one-2,3-dihydro-1H-benzo[d]imidazol-1-yl)benzene formic acid
[0227]
[0228] The concrete steps of preparation embodiment 1 compound are as follows:
[0229] (1) Preparation of 4-(2-fluoro-6-nitro-phenylamino)methylbenzoate (1-1):
[0230]
[0231] Methyl 4-aminobenzoate (302 mg, 2 mmol) was dissolved in tetrahydrofuran (THF) (20 mL) and dimethylformamide (DMF) (4 mL), and sodium hydride (NaH) (60%, 120mg, 3mmol), stirred for 10min, then added 1,2-difluoro-3-nitrobenzene (318mg, 2mmol) and stirred for 5 hours, quenched with water (10mL), extracted with ethyl acetate (50mL), and the organic phase Dry over anhydrous sodium sulfate, concentrate under reduced pressure, and separate and purify the concentrate by silica gel column chromatography (eluent: n-hexane:ethyl acetate=50:1) to obtain 4-(2-fluoro-6-nitro Phenylamino)methylbenzoate 1-1 (300 mg) as a yellow solid. Yield 52%. MS+H + =291.
[0232] (2) Prepar...
Embodiment 2
[0244] Example 2: 4-(3-(2,6-dichlorobenzoyl)-7-methyl-2-one-2,3-dihydro-1H-benzo[d]imidazol-1-yl) benzoic acid
[0245]
[0246] The compound of this example can be prepared according to the steps similar to the previous example 1, the difference is that in step 1, 1-methyl-2-fluoro-3-nitrobenzene is used as the raw material instead of 1,2-difluoro- 3-nitrobenzene. Relevant characterization data are as follows: MS+H + = 441.7; 1 H-NMR (CDCl 3 , 300MHz) δ:ppm 8.36(d, J=8.1Hz, 1H), 8.22(d, J=8.4Hz, 2H), 7.51(d, J 1 =8.4Hz, 1H), 7.37~7.19(m, 4H), 7.06(d, J=8.1Hz, 1H), 1.87(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com