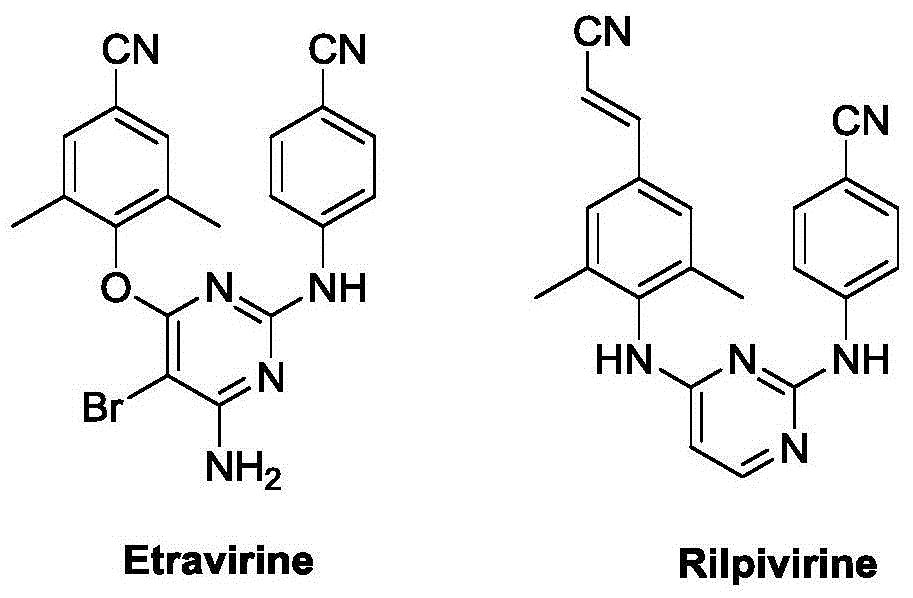
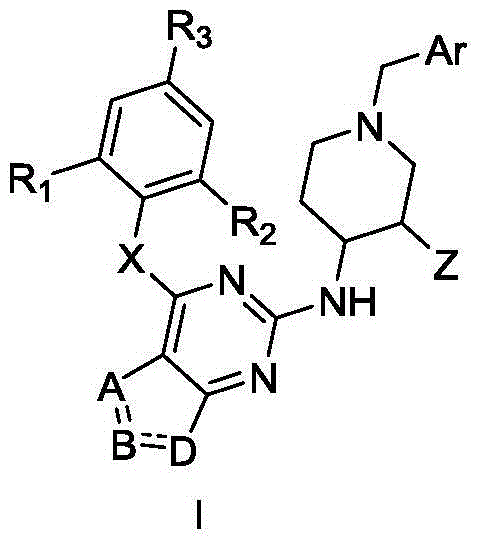
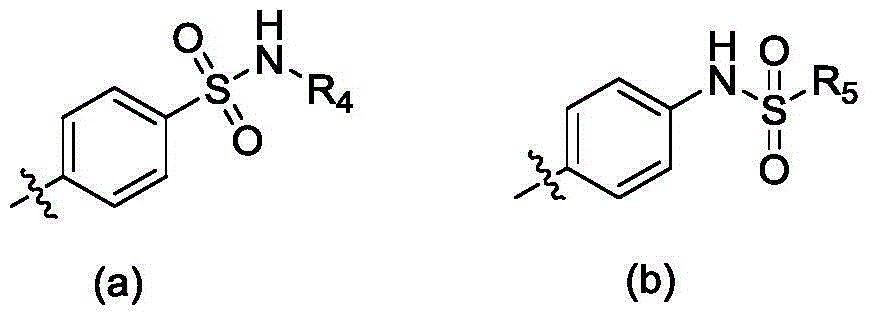
Thieno miazines derivatives and preparation method and application thereof
A technology of pyrimidine and derivatives, applied in the field of organic compound synthesis and medical application, can solve the problems of poor water solubility and low oral bioavailability, and achieve the effect of high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: 4-((2-(piperidin-4-amine)thieno[3,2-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (10) preparation
[0054]
[0055] Weigh 4-hydroxy-3,5-dimethylbenzonitrile (0.15g, 1mmol) and potassium carbonate (0.17g, 1.2mmol) in 5mL of N,N-dimethylformamide (DMF) solution, room temperature Stir for 15 minutes, then add 2,4-dichlorothieno[3,2-d]pyrimidine (0.21g, 1mmol) and continue to stir at room temperature for 2h (TLC detects that the reaction is complete). At this time, a large amount of white solid was generated, and 25mL of ice water was slowly added thereto, filtered, and dried in a vacuum oven to obtain a white solid which was the compound 4-((2-chlorothieno[3,2-d]pyrimidine- 4-yl)oxy)-3,5-dimethylbenzonitrile, yield 93.8%, melting point 258-260°C.
[0056] Weigh the compound 4-((2-chlorothieno[3,2-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (0.32g, 1.0mmol), N-Boc- 4-Aminopiperidine (0.24g, 1.2mmol) and potassium carbonate (0.28g, 2mmol) were dissolved...
Embodiment 2
[0058] Example 2: Preparation of 2-(piperidin-4-amine)-4-(2,4,6-trimethylphenoxy)thieno[3,2-d]pyrimidine (13)
[0059]
[0060] The operation steps are the same as in Example 1, except that the starting material is 2,4,6-trimethylphenol.
[0061] The product is a white solid. Yield 87.5%, 178-180°C.
[0062] 1 H NMR (400MHz, CDCl 3 -d6,ppm)δ:7.73(d,1H,J=5.4Hz,C 6 -thienopyrimidine-H), 7.19 (d, 1H, J=5.3Hz, C 7 -thienopyrimidine-H),6.90(s,2H,C 3 ,C 5 -Ph”-H), 4.89(d, 1H, J=7.4Hz, NH), 3.77(s, 1H, NH), 3.20(d, 2H, J=12.6Hz), 2.74-2.76(m, 2H) ,2.32(s,3H,C 4 -Ph"-CH 3 ),2.04-2.09(m,9H),1.46-1.54(m,2H).ESI-MS: m / z 369.5(M+1)C 20 h 24 N 4 OS(368.17)
Embodiment 3
[0063] Embodiment 3: the preparation of compound IA-1
[0064] Weigh compound 4-((2-(piperidin-4-amine)thieno[3,2-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (10) (0.5 mmol) in 10mL DMF, stirred and dissolved at room temperature, then added anhydrous potassium carbonate (0.14g, 1.0mmol) and substituted benzyl chloride or benzyl bromide (0.6mmol), stirred at room temperature for 12h (reaction completed by TLC). The solvent was distilled off under reduced pressure, then 30ml of ethyl acetate was added to the residual substrate, washed with saturated saline solution 3 times, 10mL each time, the organic layer was separated, dried over anhydrous sodium sulfate, filtered and concentrated. The target compound was separated by flash column chromatography, and then recrystallized in ethyl acetate-petroleum ether system to obtain the target compound IA-1.
[0065] With different substituted benzyl and 4-((2-(piperidin-4-amine)thieno[3,2-d]pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com