Defensin and application thereof in preparation of medicines for resisting aspergillus
An anti-Aspergillus and drug technology, applied in the direction of antifungal agents, pharmaceutical formulations, genetic material components, etc., can solve the problems of unsatisfactory treatment effect and low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Embodiment 1. Expression vector construction method of Chlorella ellipsoides nitrate reductase mutant
[0101] Primers were designed according to the Ubiquitin promoter sequence (SEQ ID NO: 1), upstream primer: 5' CCGGAAGCTT GTGCAGCGTGACCCG3' (SEQ ID NO: 2); downstream primer: 5' GCCCGGATCC CTGCAGAAGT3' (SEQ ID NO: 3), wherein a Hind III restriction site (underlined part) is added to the upstream primer, and a BamH I restriction site (underlined part) is added to the downstream primer, and PCR method is used to obtain The Ubiquitin promoter was obtained in the maize genome, and the sequencing results showed that it was a Ubiquitin promoter sequence (SEQ ID NO: 4, which added Hind III restriction sites and BamH I respectively at the 5' end and 3' end of SEQ ID NO: 1 Restriction sites). The PCR reaction conditions were: pre-denaturation at 94°C for 3 min, denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 2 min, and extension at 72°C for ...
Embodiment 2
[0102] Example 2. Using the pGreen0029 framework to construct an expression vector.
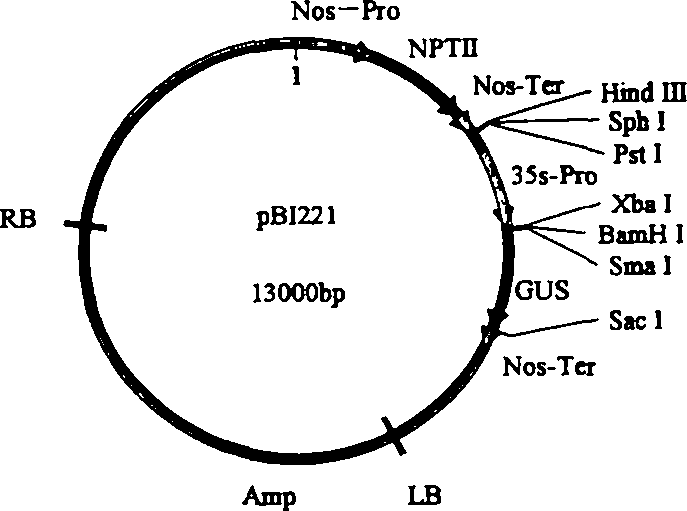
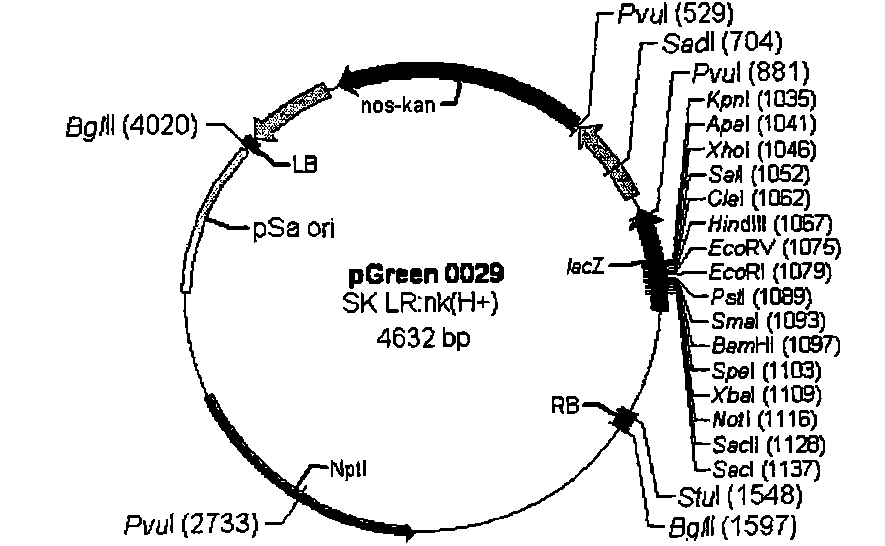
[0103] Design primers according to Nos terminator sequence (SEQ ID NO: 6), upstream primer: 5' ATAAGAATGCGGCCGC TCGAATTTCCCCGATCGTTCAAAC3' (SEQ ID NO: 7) downstream primer: 5' CGAGCTCGCCCGATCTAGTAACATAGATGA3' (SEQ ID NO: 8) wherein a Not I restriction site (underlined part) is added to the upstream primer, a Sac I restriction site (underlined part) is added to the downstream primer, and the PCR method is used The Nos terminator was obtained from pBI221 (Clontech). The PCR reaction conditions were: pre-denaturation at 94°C for 3 min, denaturation at 94°C for 30 s, annealing at 56°C for 30 s, extension at 72°C for 30 s, and extension at 72°C for 10 min after 30 cycles. The 268bp fragment of the PCR product was double-digested with Not I and Sac I endonucleases, and the plasmid pGreen0029( image 3 , from BBSRC) were also double-digested with Not I and Sac I endonucleases, and then the two d...
Embodiment 3
[0104] Example 3. Construction of the expression vector pGreen-NR-U-mNP1 of Chlorella ellipsoides nitrate reductase mutant.
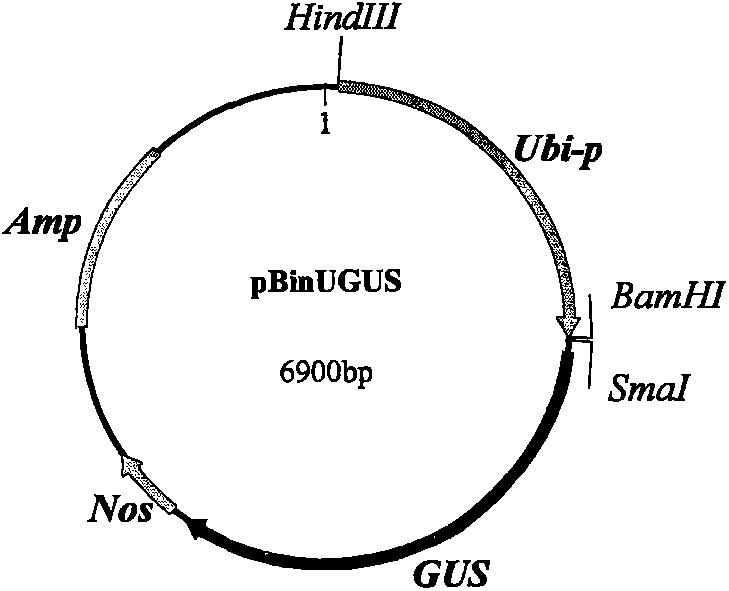
[0105] According to conventional techniques, the Ubiquitin promoter (SEQ ID NO: 1) was extracted from the vector pbinUGUS ( figure 2 ) after being recovered by HindIII and BamHI double enzyme digestion, connected to the pGreen0029nos vector that was also subjected to HindIII and BamHI double enzyme digestion ( Figure 4 ) to obtain pGreen0029-U-nos primary intermediate vector ( Figure 5 ). The sequence with the target gene mNP-1 (its sequence is as SEQ ID NO: 13) was synthesized by chemical synthesis method. In order to improve the antiviral activity of NP-1, in the process of synthesis, the N-terminal of NP-1 added A methionine transforms NP-1 into mNP-1. Because the mNP-1 gene is too small, in order to facilitate the construction of the vector, a 75bp auxiliary sequence was added to the C-terminus of the gene. Two restriction sites BamH I and Not...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com