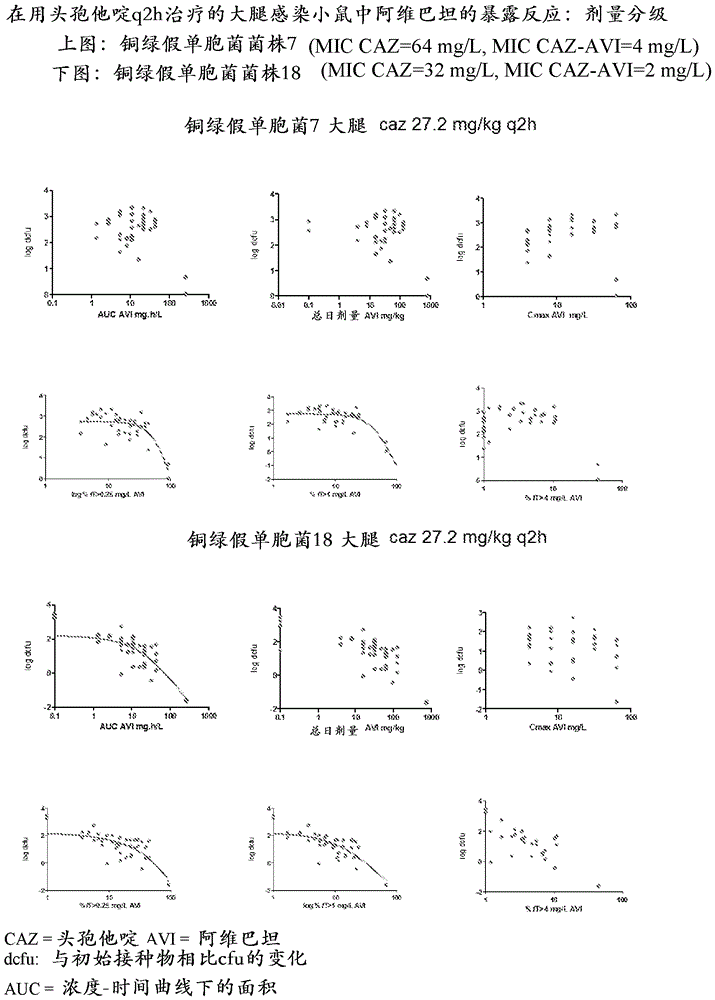
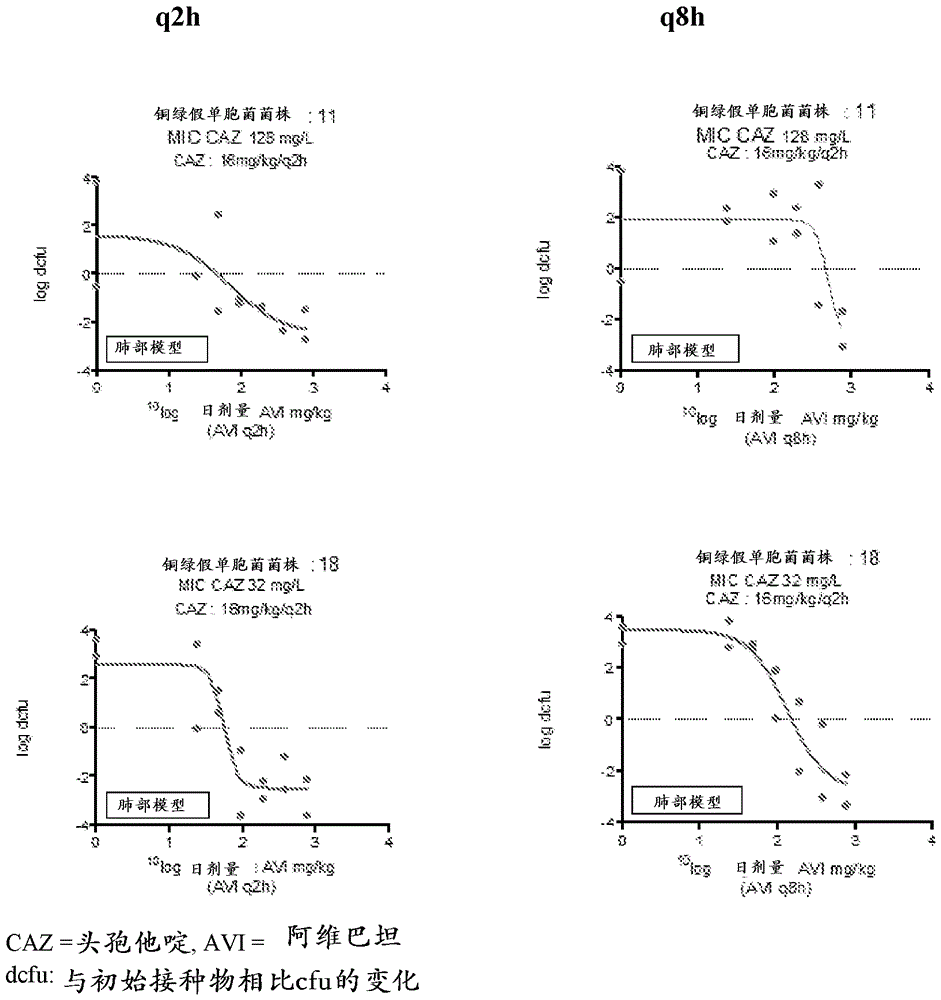
Combination therapy for the treatment of nosocomial pneumonia
A technology for acquired pneumonia and hospitals, which is applied in the direction of drug combinations, pharmaceutical formulas, organic active ingredients, etc., and can solve problems such as reduced efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 - In vitro potency of CAZ-AVI in pulmonary surfactant
[0051] bacterial strain
[0052] The bacterial strains used in this test are part of a microbial culture collection housed at AstraZeneca R&D Boston (AstraZeneca Research Collection, known as ARC). The panel of bacterial isolates used for this test contained 5 CLSI QC reference strains and the remainder were recent clinical isolates expressing β-lactamase or isolates from the primary bacterial screening panel.
[0053] Research design
[0054] MIC values were determined using the CLSI broth microdilution method with small changes. Prepare a stock compound master, and using said master, use a Perkin-Elmer MiniTrak TM The multi-position dispenser dispenses 2 µL aliquots of the 2-fold serial drug dilutions onto columns 1-11 of the 96-well subplate. Column 12 contained no drug and served as a growth control. Use multiple channels 100μL (5×10E 5 CFU / mL) Inoculum volumes in CAMHB containing 0, 1%, 2...
Embodiment 2
[0073] Example 2 - Potential drug interactions with other commonly co-administered agents
[0074] Use a checkerboard assay to test (if present) ceftazidime and ceftazidime-avibactam combinations with 6 established antimicrobials: tobramycin, levofloxacin, vancomycin, linezolid, tigecycline, and colistin interaction between. The MICs of ceftazidime and ceftazidime-avibactam in the presence and absence of various concentrations of these antibacterial agents were compared to generate a series of fractional inhibitory concentration index (FICI) values. The average FICI was taken from each combined board and interpreted according to accepted standards. In cases where antimicrobials were ineffective (vancomycin and linezolid against Gram-negative isolates; colistin against Gram-positive isolates), the individual MICs of ceftazidime and ceftazidime-avibactam were compared with all The above MICs are related to the C of these antimicrobials 最大 and 0.5×C 最大 combination for compa...
Embodiment 3
[0114] Example 3 - CAZ-AVI penetration into ELF
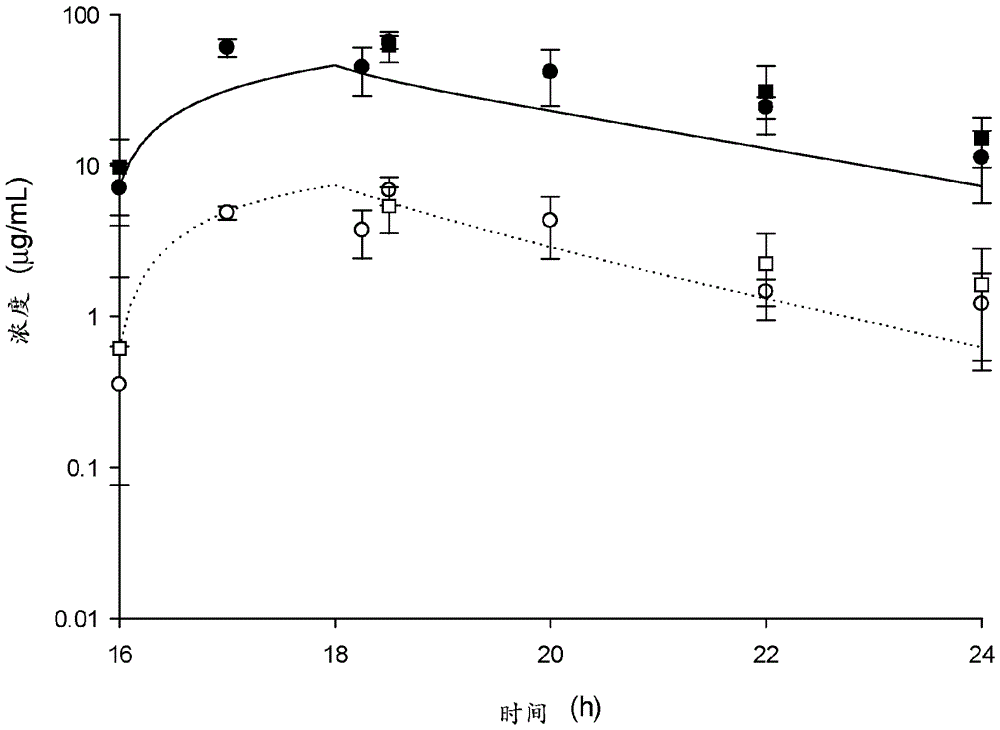
[0115] Pharmacokinetic studies were performed to describe the pulmonary disposition of ceftazidime-avibactam in infected and uninfected mice. Then, the efficacy of ceftazidime and ceftazidime-avibactam against Pseudomonas aeruginosa isolates was studied using a neutropenic lung infection model. No pharmacokinetic differences were observed in serum or ELF between infected and uninfected mice. Using human mock serum doses of 2000 mg ceftazidime and 500 mg avibactam as a 2-h infusion, maximal activity against these isolates was observed at an MIC of 32 μg / mL, where for the upper 95% confidence interval ELF fT > MIC ≥ 19%. Given the MIC of ceftazidime-avibactam 90 At 8 μg / mL, the MIC was higher for few isolates, and even fewer isolates were able to grow in the murine model of lung infection. Therefore, a ceftazidime directed ELF fT > MIC study was performed and showed activity against an MIC of 32 μg / mL with an ELF fT > MIC o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com