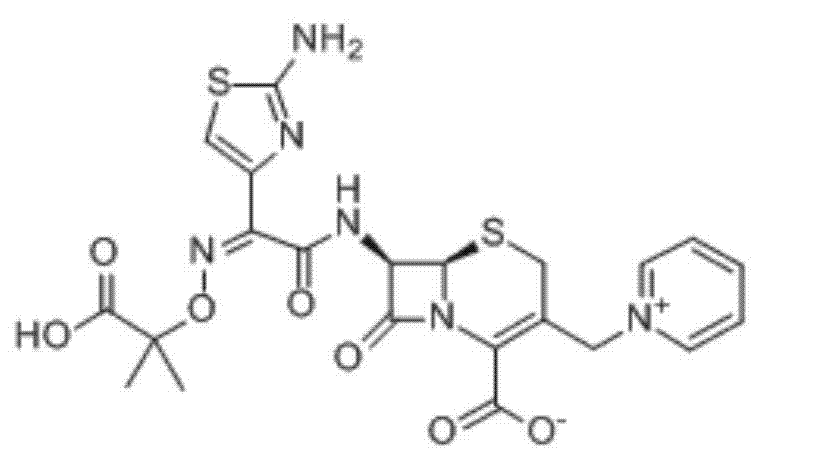
Ceftazidime composition for injection and preparation method for ceftazidime composition
A technology of ceftazidime and composition, which is applied in the field of ceftazidime for injection and its preparation field, can solve the problems of easy occurrence of adverse reactions, poor stability and change of the composition at risk of drug use by patients, etc., so as to enhance the effect of solubilization and improve the heart and brain. The effect of blood supply and avoiding shortness of breath
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
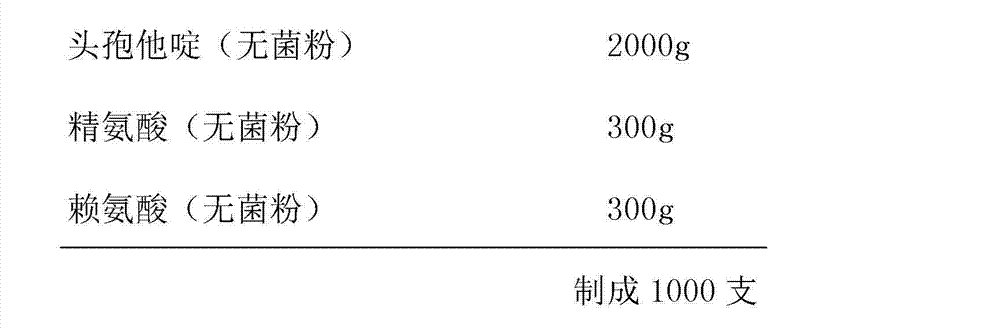
[0037] Prescription (specification: 2.0g):
[0038]
[0039] Preparation Process:
[0040] Sterilize the outer barrels of ceftazidime, arginine and lysine with 75% ethanol, and then irradiate them with ultraviolet light for more than 30 minutes. Sieve, then weigh the above-mentioned 3 kinds of materials of prescription quantity, set aside; Add ceftazidime, lysine, arginine to three-dimensional aseptic powder kinematic mixer successively, fully mix under rotating speed 30rmp-40rmp, 2-3 hours; Put the ceftazidime mixed powder into the filling machine, adjust the filling volume according to the batch production instructions, put it into the bottle after passing the inspection, and buckle the butyl rubber stopper; get it after crimping, light inspection, and packaging; among them, the powder is mixed and packed It is carried out under Class A clean area.
Embodiment 2
[0042] Prescription (specification: 2.0g):
[0043]
[0044] Preparation Process:
[0045] Sterilize the outer barrels of ceftazidime, arginine and lysine with 75% ethanol, and then irradiate them with ultraviolet light for more than 30 minutes. Sieve, then weigh the above-mentioned 3 kinds of materials of prescription quantity, set aside; Add ceftazidime, lysine, arginine to three-dimensional aseptic powder kinematic mixer successively, fully mix under rotating speed 30rmp-40rmp, 2-3 hours; Put the ceftazidime mixed powder into the filling machine, adjust the filling volume according to the batch production instructions, put it into the bottle after passing the inspection, and buckle the butyl rubber stopper; get it after crimping, light inspection, and packaging; among them, the powder is mixed and packed It is carried out under Class A clean area.
Embodiment 3
[0047] Prescription (specification: 2.0g):
[0048]
[0049] Preparation Process:
[0050] Sterilize the outer barrels of ceftazidime, arginine and lysine with 75% ethanol, and then irradiate them with ultraviolet light for more than 30 minutes. Sieve, then weigh the above-mentioned 3 kinds of materials of prescription quantity, set aside; Add ceftazidime, lysine, arginine to three-dimensional aseptic powder kinematic mixer successively, fully mix under rotating speed 30rmp-40rmp, 2-3 hours; Put the ceftazidime mixed powder into the filling machine, adjust the filling volume according to the batch production instructions, put it into the bottle after passing the inspection, and buckle the butyl rubber stopper; get it after crimping, light inspection, and packaging; among them, the powder is mixed and packed It is carried out under Class A clean area.
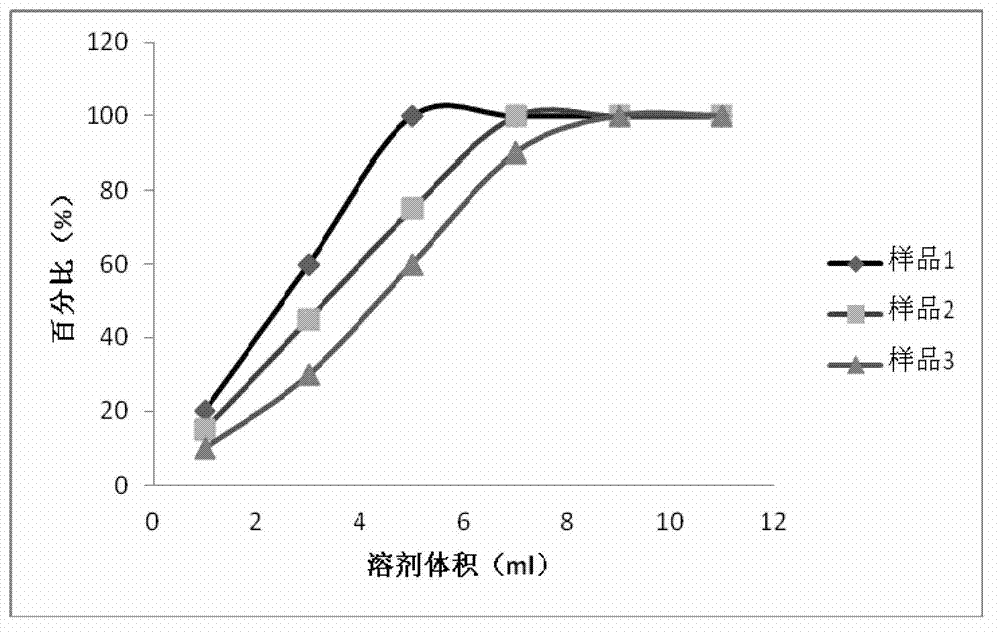
[0051] The present invention further provides the following test examples to illustrate the technical solution of the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com