A kind of production process of recovering potassium chloride from saturated potassium mirabilite mother liquor
A production process, the technology of potassium mirabilite, applied in the direction of alkali metal chloride, etc., can solve the problems of difficult separation and complex recovery process of potassium salt, and achieve the effect of simple composition, simple process and simplified precipitation form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
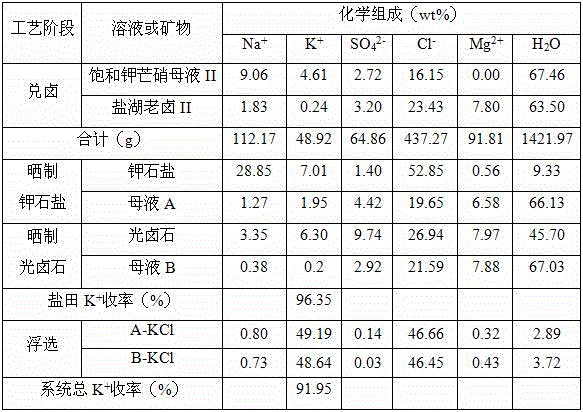
[0034] (1) Add brine: mix 1000g of saturated potassium Glauber's salt mother liquor (See Table 1 for the chemical composition) and 689g salt lake old brine (See Table 1 for the chemical composition) Mix the brine at 20°C and mix evenly to obtain 1689g of the brine mother liquor;
[0035] (2) Sun-dried potash salt: transfer 1689g of the bittern mother liquor obtained in step (1) into the potash salt field, and at 23°C, naturally evaporate 570g (equivalent to 33.75wt% of the bittern mother liquor) of water to precipitate potash Solid-liquid separation after salt, to obtain 347g potassium halite and 772g mother liquor A;
[0036] (3) Sun-dried carnallite: transfer 772g of mother liquor A obtained in step (2) into a carnallite salt field, and naturally evaporate 98g (equivalent to 12.69wt% of mother liquor A) of water at 22°C to precipitate carnallite After solid-liquid separation, 216g carnallite and 458g mother liquor B were obtained;
[0037] (4) Extraction of potassium chlo...
Embodiment 2
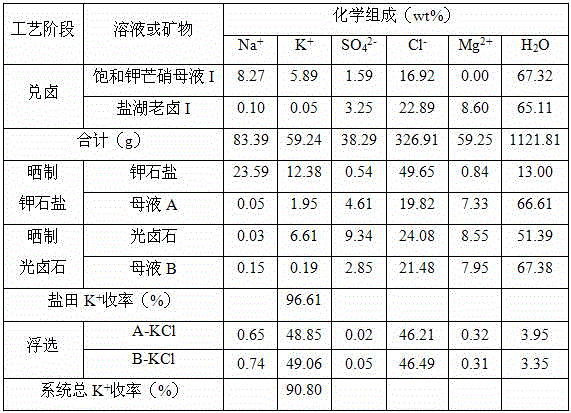
[0042] (1) Add brine: mix 1000g of saturated potassium Glauber's salt mother liquor (See Table 2 for the chemical composition) and 1177g salt lake old brine (See Table 2 for the chemical composition) Mix the brine at 25°C and mix evenly to obtain 2177g of the brine mother liquor;
[0043] (2) Potassium salt production by drying: transfer 2177g of the bittern mother liquor obtained in step (1) into the potash salt field, and at 23°C, naturally evaporate 496g (equivalent to 22.78wt% of the bittern mother liquor) of water to precipitate potassium stone Solid-liquid separation after salting, to obtain 313g potassium halite and 1368g mother liquor A;
[0044] (3) Sun-dried carnallite: transfer 1368g of mother liquor A obtained in step (2) into a carnallite salt field, and naturally evaporate 232g (equivalent to 16.96wt% of mother liquor A) of water at 26°C to precipitate carnallite After solid-liquid separation, 400g carnallite and 736g mother liquor B were obtained;
[0045] ...
Embodiment 3
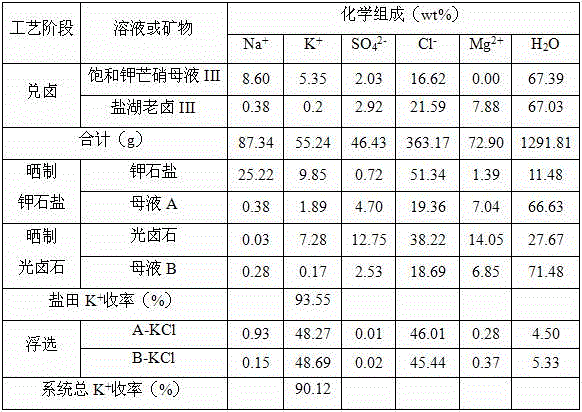
[0050] (1) Add brine: mix 1000g of saturated potassium Glauber's salt mother liquor (See Table 3 for the chemical composition) and 917g salt lake old brine (See Table 3 for the chemical composition) Mix the brine at 30°C and mix evenly to obtain 2917g of the brine mother liquor;
[0051] (2) Potassium salt production by drying: transfer 1917g of the bittern mother liquor obtained in step (1) into the potash salt field, and naturally evaporate 562g (equivalent to 29.32wt% of the bittern mother liquor) of water at 28°C to precipitate potassium stone Solid-liquid separation after salt, to obtain 340g potassium halite and 1015g mother liquor A;
[0052] (3) Sun-dried carnallite: transfer 1015g of mother liquor A obtained in step (2) into a carnallite salt field, and naturally evaporate 242g (equivalent to 23.84wt% of mother liquor A) of water at 27°C to precipitate carnallite After solid-liquid separation, 251g carnallite and 522g mother liquor B were obtained;
[0053] (4) E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com